(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
No fee required. | |||||
o | Fee computed on table below per Exchange Act Rules 14a-6(i) | ||||
| 1) | Title of each class of securities to which transaction applies: | ||||
| 2) | Aggregate number of securities to which transaction applies: | ||||
| 3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | ||||
| 4) | Proposed maximum aggregate value of transaction: | ||||
| 5) | Total fee paid: | ||||
o | Fee paid previously with preliminary | ||||
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the | ||||
| 1) | Amount previously paid: | ||||
| 2) | Form, Schedule or Registration Statement No.: | ||||
| 3) | Filing Party: | ||||
| 4) | Dated Filed: | ||||

PROXY STATEMENT
AND NOTICE OF ANNUAL
SHAREHOLDER MEETING
2021

PLEASE VIEW OUR 2020 ANNUAL REPORT
investor.regeneron.com/2020AR

PLEASE VIEW OUR 2020 RESPONSIBILITY REPORT
investor.regeneron.com/2020RR


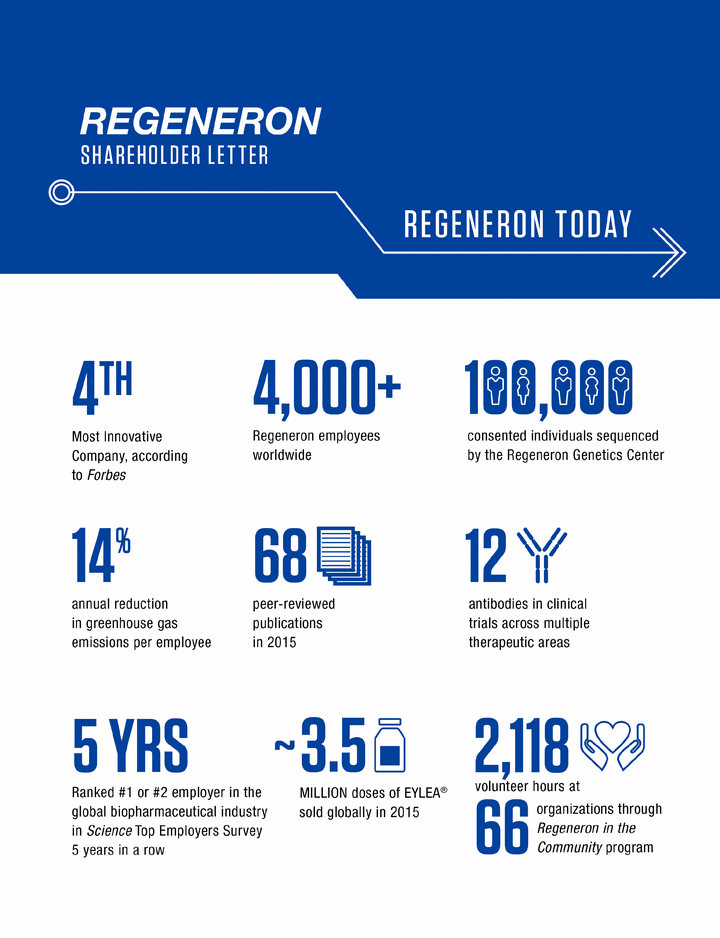
2020 BY THE NUMBERS
 in clinicaldevelopment |
| 41 countries where weconducted clinical trials | 6 U.S. marketing applicationsfor new products or new indications for existing products |
| $3.9 million raised fromemployee donations and company matches – nearly four times previous years | 179 manuscriptspublished in peer-reviewed journals | 100+ RegeneronGenetics Center collaborations in 21 countries | Provided STEMexperiences to 524,000 students |

LETTER TO SHAREHOLDERS
DEAR FELLOW SHAREHOLDERS,
When we wrote to you this time last year, the novel coronavirus, SARS-CoV-2, had recently been declared a global pandemic. Our team had quickly identified ways Regeneron could help and had already begun isolating novel antibodies to combat the disease, but no one recognized the epic, world-changing challenges COVID-19 and 2020 would bring. The numbers have been sobering – nearly 100 million people infected globally, several million dead, and almost everyone impacted in some significant way. The Regeneron team has been deeply impacted as well, from an early outbreak near our headquarters in Westchester, New York, to the personal loss of loved ones.
Despite this unprecedented public health crisis, 2020 was an inspiring year in many ways, demonstrating the power of science and the resilience of our team. We discovered, developed, and manufactured our novel REGEN-COV™ (casirivimab with imdevimab) antibody cocktail treatment for COVID-19 in record time – just 10 months from program inception through an emergency use authorization (“EUA”) from the U.S. Food and Drug Administration (“FDA”). To date, tens of thousands of patients have received REGEN-COV, and we are now working in partnership with the U.S. government, healthcare providers, and advocacy groups to ensure all appropriate patients can access it.
Unlike vaccines, which trigger the body’s own immune response to protect against infection, REGEN-COV provides virus neutralizing antibodies directly to the patient. In the pivotal Phase 3 treatment trial, REGEN-COV reduced hospitalization or death by 70% in high-risk outpatients and reduced symptom duration. As we do our part to bring this pandemic to an end, we continue to evaluate REGEN-COV in additional patient populations, at lower dose levels, and for prevention purposes. To that end, results from the Phase 3 prevention trial showed that REGEN-COV administered as a subcutaneous injection reduced the risk of symptomatic SARS-CoV-2 infections by 81% among household contacts of infected patients. As of April 2021, more than 25,000 people have participated in clinical trials of REGEN-COV, and we thank all the individuals, investigators, and collaborators.
Our financial position remained strong this year, with top-line growth of 30% and bottom-line growth of 28%1 through an increasingly diversified set of revenue and earnings streams. Total revenues for 2020 increased to $8.5 billion, compared to $6.6 billion for the full year 2019.
EYLEA® (aflibercept) Injection continues to reach more patients in competitive eye disease markets, with its efficacy, safety, and convenience setting a high bar for current and potential future entries. We are confident in the durability and continued growth of this important medicine for years to come. Annual EYLEA global net product sales reached nearly $8 billion in 2020 (net product sales outside the U.S. recorded by our collaborator Bayer), and $4.9 billion in the U.S., still without a single price increase in its history.
Looking to the rest of our growing portfolio, more than 80% of our top-line growth in 2020 came from products and revenues other than EYLEA. Dupixent® (dupilumab) global net product sales in 2020 (recorded by our collaborator Sanofi) were more than $4 billion, reflecting growth of 75% versus 2019. This “pipeline in a product” continues to reach more patients in need with an expanded FDA indication for atopic dermatitis in patients ages 6 to 11 and an FDA acceptance of our supplemental application as an add-on treatment for children aged 6 to 11 years with uncontrolled moderate-to-severe asthma, with even more room to grow as it meets its potential to transform the treatment of certain type 2 inflammatory diseases. We also made Dupixent treatment more convenient with the FDA approval of a single-dose, 300mg pre-filled syringe.
As the foundation of our oncology portfolio, our PD-1 inhibitor Libtayo® (cemiplimab) is achieving significant and steady growth with FDA approvals in two new indications, non-small cell lung cancer and basal cell carcinoma, in early 2021. Global net product sales for Libtayo were $348 million in 2020, representing 80% year-over-year growth. We are making progress in other cancers as well, including in March 2021 when positive results in overall survival prompted us to stop our cervical cancer trial early, with the data forming the basis of upcoming regulatory submissions. With 11 investigational therapeutics in clinic for a wide range of cancers, including eight bispecific antibodies, we continue to diversify our approach to oncology and are positioned to lead the next wave of innovation in immuno-oncology.
| 1 | Bottom-line growth represented by non-GAAP net income per share – diluted, which is not a measure calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). See Appendix A for a definition of this measure and a reconciliation of this measure to the most directly comparable GAAP financial measure. |
 | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |

Our COVID-19 program and other important progress this year was made possible by decades of investment in our foundational VelociSuite® antibody discovery and development technologies, as well as in world-class manufacturing enterprise. Thanks to these investments and the hard work of our colleagues, in 2020 and early 2021 we achieved two new FDA-approvals of novel, Regeneron-discovered antibody medicines: the multi-antibody cocktail Inmazeb™ (atoltivimab, maftivimab, and odesivimab-ebgn) for Ebola, and the ANGPTL3 inhibiting antibody Evkeeza™ (evinacumab) for a rare form of inherited high cholesterol.
Regeneron is known for our science-driven approach, and as such our pipeline and research efforts continue to expand. We continue to reinvest a significant portion of our growing revenue into our R&D efforts to fuel the remarkable innovation and curiosity of our world-class team. Our early pipeline is increasingly powered by genetics, thanks to significant insights from the Regeneron Genetics Center®, which reveals new targets for exploration as well as enriching current clinical programs. Our genetics medicine efforts also include important collaborations with Intellia Therapeutics, Inc. and Alnylam Pharmaceuticals, Inc., which pair Regeneron’s biologic and antibody capabilities with cutting-edge technologies like CRISPR gene editing and RNA silencing. Both of these partnerships advanced candidates into clinical development for the first time in the past year.
While 2020 tested us in new ways, we are proud to say that the Regeneron team successfully advanced our mission of using the power of science to bring new medicines to people in need. We came together as never before. Watching our employees rally to support each other was awe-inspiring, as was the strong spirit of collaboration and pride in our collective purpose. We head into this next year with the confidence that we will continue to tackle some of the world’s biggest health and scientific challenges.
Sincerely,
 |  |  |
P. Roy Vagelos, Chairman of the Board | Leonard S. Schleifer, President and | George D. Yancopoulos, President and |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |

| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
![]()
REGENERON PHARMACEUTICALS, INC.
777 Old Saw Mill River Road
Tarrytown, New York 10591-6707
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
The 20162021 Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. (the "Company"“Company”) will be held on Friday, June 10, 2016,11, 2021, commencing at 10:30 a.m., Eastern Time, virtually via the Internet and, if required by New York law (as discussed below), at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York, for the following purposes:
(1)to elect three Class I directors for a term of three years;(2)to ratify the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016; and(3)to act upon such other matters as may properly come before the meeting and any adjournment(s) or postponement(s) thereof.
| 1 | to elect four Class III directors for a three-year term; |
| 2 | to ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2021; and |
| 3 | to act upon such other matters as may properly come before the meeting and any adjournment(s) or postponement(s) thereof. |
The board of directors has fixed the close of business on April 14, 201613, 2021 as the record date for determining shareholders entitled to notice of, and to vote at, the Annual Meeting and at any adjournment(s) or postponement(s) thereof.
Pursuant to the rules of the Securities and Exchange Commission (the “SEC”), we have elected to use the "Notice“Notice and Access"Access” method of providing our proxy materials over the Internet. Accordingly, we will mail, beginning on or about April 27, 2016,23, 2021, a Notice of Internet Availability of Proxy Materials to our shareholders of record and beneficial owners as of the record date (other than (i) those who previously elected to access thereceive proxy materials over the Internet,by e-mail, (ii) those who have previously asked to receive paper copies of the proxy materials, and (iii) shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan). As of the date of mailing of the Notice of Internet Availability of Proxy Materials, all shareholders and beneficial owners will have the ability to access all of the proxy materials on a website referenced in the Notice of Internet Availability of Proxy Materials.
The Notice of Internet Availability of Proxy Materials also contains a toll-free telephone number, an e-mail address, and a website where shareholders can request a paper or electronic copy of the proxy statement, our 20152020 annual report, and/or a form of proxy relating to the Annual Meeting. These materials are available free of charge. The Notice also contains information on how to access and vote the form of proxy.
Due to continuing concerns regarding the COVID-19 pandemic and to assist in protecting the health and well-being of our shareholders, directors, and employees, we have opted to hold the Annual Meeting as a virtual-only meeting. Shareholders will be able to attend the Annual Meeting and participate electronically, which will allow them to vote their shares on the date of the Annual Meeting and ask questions during the meeting. Under New York law, the legal requirement to include an in-person option has been waived by relevant governmental action. If this waiver is no longer in effect for the Annual Meeting, shareholders will have the option to attend the Annual Meeting in person at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York (or at another venue if required by the circumstances). In any such case, we would notify our shareholders in advance on our website and by issuing a press release and filing it as additional proxy material with the SEC. Please visit our website at http://newsroom.regeneron.com for the most up-to-date information on the Annual Meeting, including information regarding any government-imposed limits on public gatherings or any procedures and limitations concerning in-person attendees applicable to the Annual Meeting that may be in effect at that time.
As Authorized by the Board of Directors,

Joseph J. LaRosa
Executive Vice President, General Counsel and Secretary
April 23, 2021
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
 |

April 26, 2016
REGENERON PHARMACEUTICALS, INC.
![]()
TABLE OF CONTENTS
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / i / i |
TABLE OF CONTENTS (CONT.)
i
Table of Contents
Note Regarding Forward-Looking Statements and Non-GAAP Financial Measures
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND NON-GAAP FINANCIAL MEASURES: See Appendix A for important information regarding forward-looking statements and financial measures not calculated in accordance with U.S. Generally Accepted Accounting Principles contained in this proxy statement.
ii
TableNOTE REGARDING TRADEMARKS AND PRODUCT NAMES: “ARCALYST®,” “Evkeeza™,” “EYLEA®,” “Inmazeb™,” “Libtayo®” (in the United States), “Praluent®” (in the United States), “REGEN-COV™,” Regeneron®,” “Regeneron Genetics Center®,” “VelociGene®,” “VelocImmune®,” “VelociSuite®,” and “ZALTRAP®” are trademarks of Contents
![]()
Proxy SummaryRegeneron Pharmaceuticals, Inc. (“Regeneron”). This proxy statement refers to products marketed or otherwise commercialized by Regeneron, its collaborators, and other parties. Consult the product label in each territory for specific information about such products.
The summary below highlights information that is described in more detail elsewhere in this proxy statement. This summary does not contain all of the information you should consider, and we urge you to read the entire proxy statement carefully before voting.
General Information (see "General Information about the Meeting" on page 6 for more information)
ii /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
USERS' GUIDE
GENERAL INFORMATION
| Meeting Date: | Time: | Location: | Record Date: |
| 10:30 A.M., ET | ONLINE AT | APRIL 13, 2021 | |
MEETING AGENDA
Meeting Agenda
| Matter | Board Vote Recommendation | |||
| 1 | Election of | FOR each director nominee
| ||
| 2 | Ratification of the appointment of PricewaterhouseCoopers LLP as the | FOR
| ||
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 1 / 1 |
Proposal No. 1 –USERS' GUIDE / PROXY HIGHLIGHTS
WE SEEK YOUR INPUT ON OUR BOARD
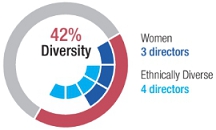
The composition of our board of directors reflects our core principle of “science first”: over half of our directors are members of the National Academy of Sciences, and our board members include two Nobel laureates and holders of many scientific awards. By having our board of directors heavily populated with top scientific talent, we signal to our shareholders and employees our seriousness about the Company’s dedication to science and its core competencies and primary value driver. Our Director Nominees (see "Proposalboard also includes individuals with experience building shareholder value through all stages of corporate development, as well as governance, financial, and policy expertise. Three of our board’s current 12 members are women, and four directors are diverse by race or ethnicity. Each of the directors who joined the board since 2016 is diverse by gender or race/ethnicity.
Please refer to “Proposal No. 1: Election of Directors" on page 10Directors” for more information)
The following individuals have been nominated for election at the 2016 Annual Meeting:
| Director Class | Name | Age* | Director Since | Occupation | Independent | Committee Memberships | ||||||
| Class I | Michael S. Brown, M.D. | 75 | 1989 | Distinguished Chair in Biomedical Sciences, Regental Professor of Molecular Genetics, and Director of the Jonsson Center for Molecular Genetics, University of Texas Southwestern Medical Center at Dallas | ü | Technology Committee (Chairman) Corporate Governance and Compliance Committee | ||||||
| Class I | Leonard S. Schleifer, M.D., Ph.D. | 63 | 1988 | President and Chief Executive Officer of Regeneron Pharmaceuticals, Inc. | | Technology Committee (Ex Officio Member) | ||||||
| Class I | George D. Yancopoulos, M.D., Ph.D. | 56 | 2001 | President, Regeneron Laboratories and Chief Scientific Officer of Regeneron Pharmaceuticals, Inc. | | Technology Committee (Ex Officio Member) | ||||||
| | | | | | | | | | | | | |
*As of April 14, 2016.
1
Proxy Summary
Each director nominee is a current director and attended at least 75% of the aggregate of all 2015 meetings of the board of directors and each committee on which he served.
Corporate Governance (see "Corporate Governance" on page 15 for more information)additional information.
Regeneron is committedWE SEEK RATIFICATION OF OUR AUDITORS
We pay close attention to good corporate governance, whichthe requirements applicable to us as a publicly traded company, including those relating to the audit of Regeneron’s financial statements by our independent registered public accounting firm, PricewaterhouseCoopers LLP. In this proxy statement, we believe promotesare asking you to ratify the long-term interests of shareholders, strengthens the accountability of the board of directors and management, and helps build trust in the Company. The following chart summarizes key information regarding our corporate governance.
*As of April 14, 2016.
Proposal No. 2 – Ratificationappointment of PricewaterhouseCoopers LLP (see "Proposal as our independent registered public accounting firm for the fiscal year ending December 31, 2021.
Please refer to “Proposal No. 2: Ratification of Appointment of Independent Registered Public Accounting Firm" on page 33Firm” for more information)
We ask that our shareholders ratify the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for 2016. Below is a summary of fees related to services provided to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 2015 and 2014.
| | 2015 | | 2014 | ||||
Audit Fees | $ | 1,721,000 | $ | 1,567,493 | |||
Audit-Related Fees | | 2,007 | | – | |||
All Other Fees | | 4,637 | | 4,812 | |||
| | | | | | | | |
Total Fees | $ | 1,727,644 | $ | 1,572,305 |
2015 Performance Overview (see "Executive Compensation – Compensation Discussion and Analysis – Section 1 – Summary – 2014 Performance Overview" on page 35 for more information)
2015 was another extraordinary year for Regeneron. Our key accomplishments in 2015 included:
•47% growth in EYLEA® (aflibercept) Injection global net product sales as compared to 2014;
•46% growth in our total revenues as compared to 2014;•19% growth in non-GAAP net income as compared to 2014 (non-GAAP net income is not a measure calculated in accordance with U.S. Generally Accepted Accounting Principles; see Appendix A for a definition of non-GAAP net income and a reconciliation of non-GAAP net income to net income);•advances in our EYLEA® franchise, including regulatory approval of EYLEA® for the treatment of visual impairment due to macular edema secondary to retinal vein occlusion and the treatment of visual impairment secondary to myopic choroidal neovascularization in the European Union; regulatory approval of EYLEA® for the treatment of diabetic retinopathy in patients with diabetic macular edema in the United States; and regulatory approval of EYLEA® for the treatment of retinal vein occlusion in Japan;•regulatory approval and launch of Praluent® (alirocumab) Injection, the first drug approved by the U.S. Food and Drug Administration ("FDA") in a new class of drugs that lower LDL ("bad") cholesterol;•positive Phase 3 data for sarilumab from three Phase 3 studies in patients with rheumatoid arthritis (SARIL-RA-TARGET, SARIL-RA-EASY, and SARIL-RA-ASCERTAIN) and submission of a Biologics License Application for sarilumab with the FDA;
2
Proxy Summary
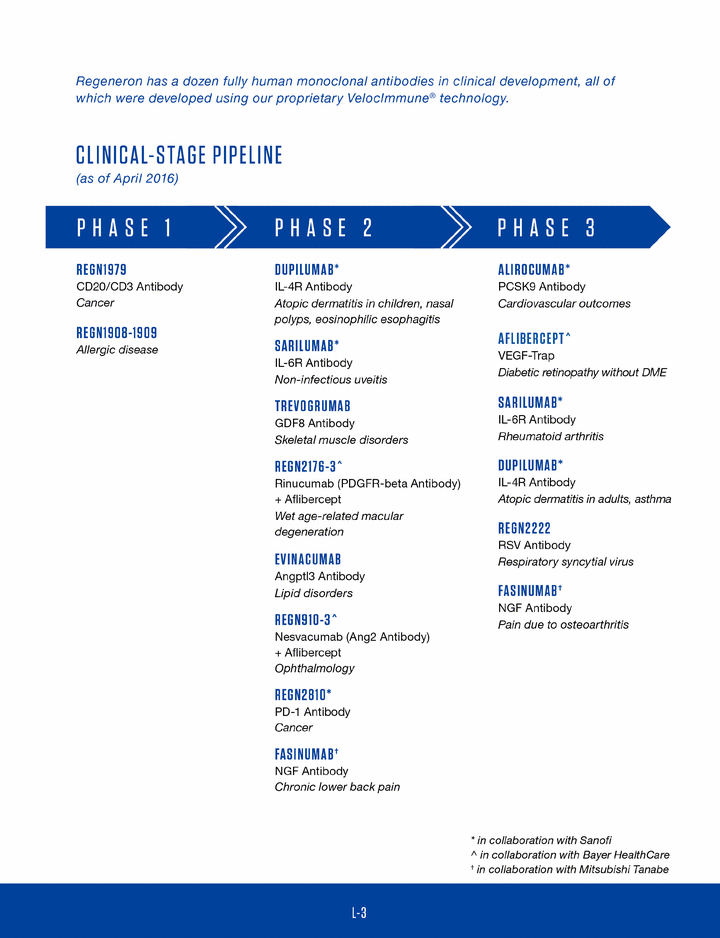
•positive pivotal Phase 2b data for dupilumab in asthma and completion of enrollment of the dupilumab atopic dermatitis Phase 3 studies;•new collaboration agreement relating to fasinumab with Mitsubishi Tanabe Pharma Corporation for Japan, Korea, and nine other Asian countries, excluding China;•initiation of Phase 3 clinical study of REGN2222 for Respiratory Syncytial Virus;•continued growth of our clinical development pipeline, as evidenced by the submission of one Investigational New Drug Application with the FDA in 2015 and 13 product candidates (consisting of one Trap-based and 12 fully-human monoclonal antibody product candidates based on the Company'sVelocImmune®technology) in clinical development as of December 31, 2015;•new global strategic collaboration with Sanofi to discover, develop, and commercialize antibody-based cancer treatments in the field of immuno-oncology; and•further important steps to support our current and future growth, including adding two new buildings in the Tarrytown campus providing nearly 300,000 square feet ofadditionallaboratory and office space; significant progress with the construction of a new manufacturing facility in Limerick, Ireland; and increasing headcount on a year-over-year basis by approximately 47% as of December 31, 2015.
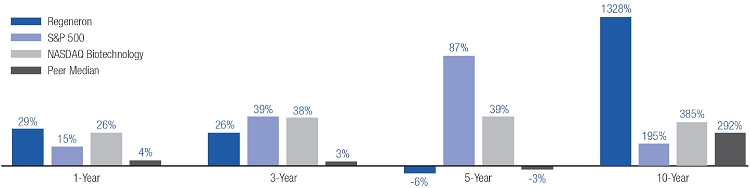
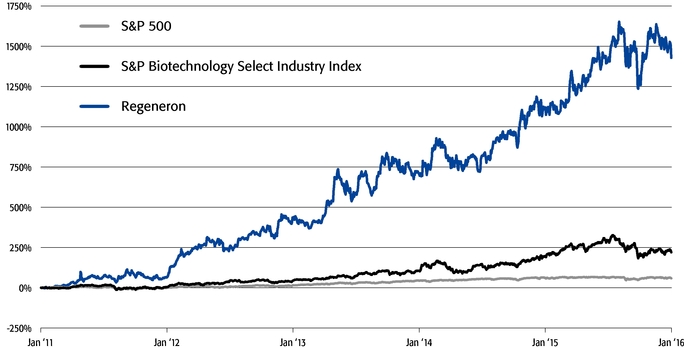
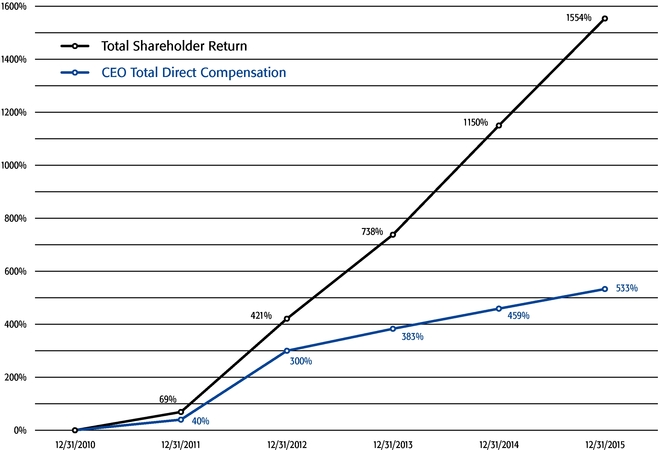
Our strong performance is reflected in the appreciation of our stock price, which increased 32%, 217%, and 1554% over the one-, three-, and five-year periods ended December 31, 2015, respectively. This shareholder return places our common stock performance in the 85th, 90th, and 99th percentile, respectively, of all NASDAQ-listed companies with a market capitalization greater than $5 billion in those periods.
Executive Compensation (see "Executive Compensation" on page 35 for more information)
We believe that the leadership of the current executive team has been instrumental to our success in 2015 and prior years, and that an executive compensation program that attracts, motivates, and helps retain key executives, including the Named Officers, is critical to our long-term success.
The main objectives of our executive compensation program are to pay for performance; closely align the interests of shareholders and management; strike a balance between short- and long-term perspectives and support our long-term growth prospects; and attract and retain highly skilled and talented executives in a competitive marketplace.information.
These objectives were reflected in our 2015 compensation decisions in a number of ways, including the following:
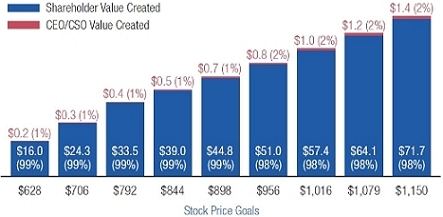
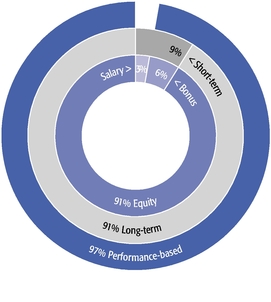
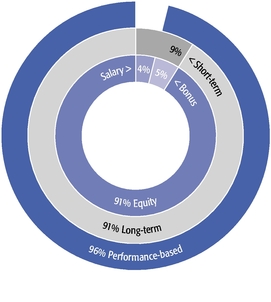
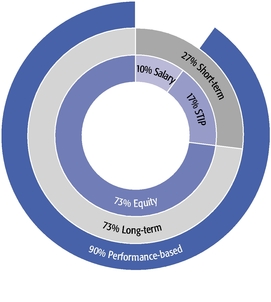
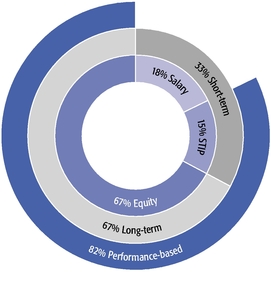
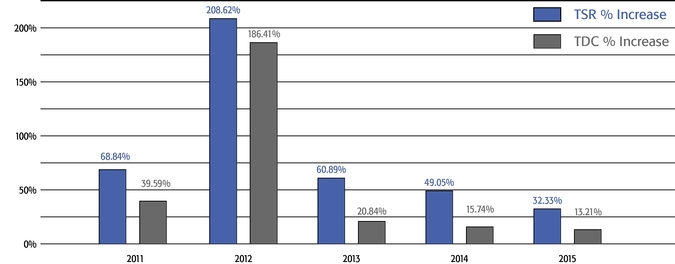
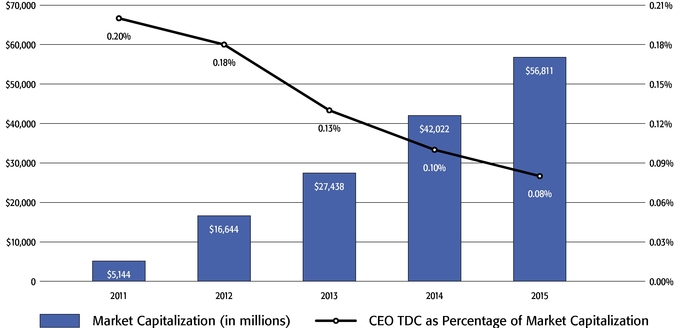
•We believe in performance-based compensation and long-term incentives.In 2015, we continued to rely primarily on performance-based compensation, both for our short-term (cash bonus) and long-term incentives (stock option awards). This emphasis on performance-based compensation (particularly long-term incentives in the form of stock options) has been a consistent part of our philosophy since Regeneron's inception, including prior to the significant appreciation in Regeneron's stock price that began in early 2011.•We believe that time-based stock options are inherently performance based, as they provide value to employees only if there is future stock price appreciation and do not provide any value to employees if the stock price declines below the exercise price.As illustrated by the charts in "Executive Compensation – Compensation Discussion and Analysis – Section 2 – Analysis of 2015 Executive Compensation Based on Compensation Objectives," this emphasis on stock options has resulted in close alignment of our Chief Executive Officer's compensation in 2015 and over the last five years with the performance of our common stock over those periods:oBoth in 2015 and over the five-year period ended December 31, 2015, the year-over-year increases in our Chief Executive Officer's compensation were principally attributable to the significant appreciation in our stock price, which increased the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model for valuing stock options.oOver the same periods, the Black-Scholes grant date fair value of stock option grants to our Chief Executive Officer increased less than the appreciation of our stock price, in part because the absolute number of stock options granted to our Chief Executive Officer decreased in the last three years. The number of shares underlying the annual stock option award to our Chief Executive Officer in 2015 was approximately 39% lower than in 2012, while the stock price appreciated 217% over the same period. As a result, the appreciation in the reported value of our Chief Executive Officer's pay was significantly below the appreciation of our stock price, both cumulatively over the five-year period and on a year-over-year basis. This means that the value of our long-term shareholders' investment in Regeneron grew more rapidly than our CEO's pay over those periods.oTo further illustrate this point, over the last five years, our Chief Executive Officer's total direct compensation, as a percentage of Regeneron's capitalization in the year in which the compensation was awarded, decreased from 0.20% to 0.08%.
3
Proxy Summary
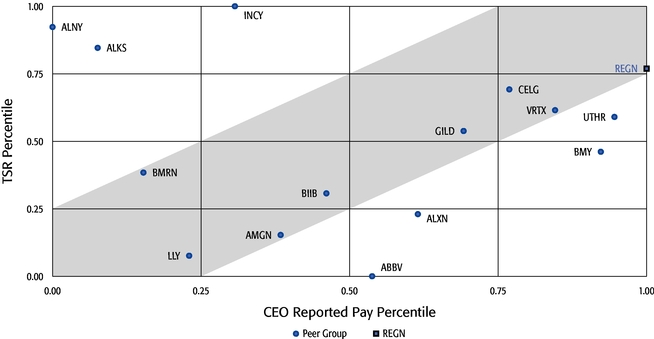
oAs a result of our emphasis on performance-based compensation, on a relative basis when compared to our Peer Group, the total direct compensation of our Chief Executive Officer over the last three years was also closely aligned with the performance of our common stock even when taking into account the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model.
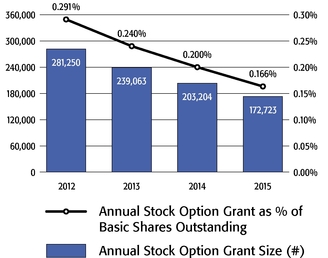
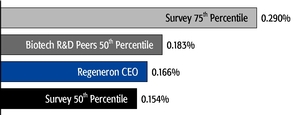
•We believe in year-over-year consistency in making compensation decisions and in striking a balance between the dilutive impact of equity grants and the competitiveness of our compensation program.In our compensation decisions, we focus on the number of shares underlying equity awards relative to the number of basic shares of common stock outstanding, rather than the grant date fair value of the award (as determined according to the Black-Scholes model). We believe this ownership- and dilution-based approach to awarding stock options provides a better measure of the amount of potential increases in shareholder value that would be shared by the awards and allows us to evaluate such grants on a consistent basis as compared to other companies and regardless of fluctuations in the price of Regeneron's or other companies' common stock. Further, focusing on the number of shares and the incremental sharing rate of potential future upside (rather than targeting a specific Black-Scholes grant date fair value) avoids rewarding officers with larger grant sizes following a decline in our stock price.oAs a percentage of the total basic shares outstanding, the 2015 stock option award to our Chief Executive Officer was significantly below the 75thpercentile of the companies included in the 2015 Radford Global Life Sciences Survey and only slightly above the 50thpercentile (at 0.166% compared to 0.290% and 0.154%, respectively). In addition, this award was below the 50thpercentile of our Biotech R&D Peers (which was 0.183%).oIn 2015, the Compensation Committee reduced the number of shares underlying the annual stock option awards to the Named Officers by 15% compared to 2014 (other than
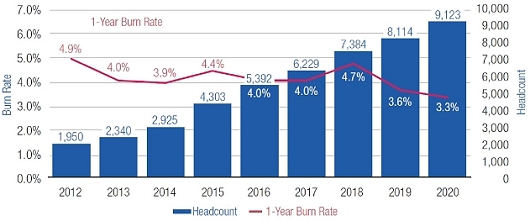
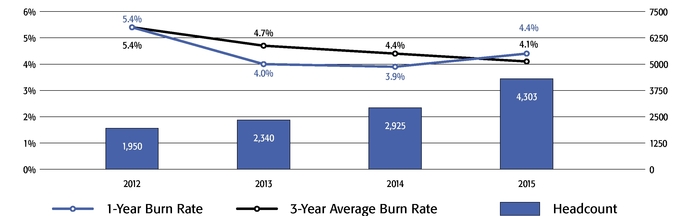
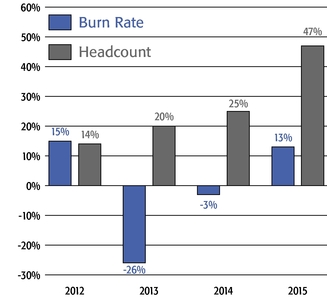
Mr. Terifay's award, which remained at the 2014 level due to his promotion to Executive Vice President, Commercial). This decrease constituted the third consecutive double-digit percentage decrease in the annual grant of stock options to our Named Officers, in each case following outstanding TSR performance. In reducing the size of 2015 annual stock option awards to executives, the Compensation Committee sought to reduce the potential dilutive impact of new equity awards without adversely affecting the competitiveness of our executive compensation program, which has successfully motivated our senior management team to deliver high operating performance and shareholder value.oWe continued to pay close attention to our burn rate. Despite the expansive growth of our employee base, which increased by 121% between 2012 and 2015 (from 1,950 full-time employees to 4,303 full-time employees), our burn rate decreased from 5.4% to 4.4% over the same period, and we maintained a three-year burn rate average of 4.1% in 2015. We achieved this reduction through implementing three consecutive double-digit percentage decreases in the number of shares underlying annual stock option awards, without eliminating the broad-based nature of our equity compensation program.oWe believe our approach to equity compensation has helped us to successfully grow and manage employee attrition, as evidenced by our 2015 employee turnover of approximately 6%, which compares favorably to the average employee turnover of approximately 18% for the life sciences sector based on the Fourth Quarter 2015 Radford Global Life Sciences Trends Report.
Our Compensation Policies and Practices
We have compensation policies and practices designed to enhance governance of our executive compensation program and to further our compensation objectives. These policies and practices include:
4
Proxy Summary
 |
5
USERS' GUIDE / Proxy SummaryGENERAL INFORMATION ABOUT THE MEETING
REGENERON PHARMACEUTICALS, INC.777 Old Saw Mill River RoadTarrytown, New York 10591-6707
April 26, 2016
![]()
General Information about the Meeting
GENERAL INFORMATION ABOUT THE MEETING
ANNUAL MEETING INFORMATION
Where and when willWhen is the 2016 Annual Meeting be held?Meeting?
The 2016
June 11, 2021
What time is the Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. ("Regeneron," "Company," "we," "us," and "our") is scheduled for June 10, 2016, commencing at Meeting?
10:30 a.m., Eastern Time,ET
Where is the Annual Meeting?
Due to continuing concerns regarding the COVID-19 pandemic and to assist in protecting the health and well-being of our shareholders, directors, and employees, the Annual Meeting will be held virtually via the Internet at www.virtualshareholdermeeting.com/REGN2021. We have designed the format of the Annual Meeting to ensure that shareholders are afforded similar rights and opportunities to participate as they would at an in-person meeting. Under New York law, the legal requirement to include an in-person option has been waived by relevant governmental action. If this waiver is no longer in effect for the Annual Meeting, shareholders will have the option to attend the Annual Meeting in person at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York 10591.(or at another location if required by the circumstances). In any such case, we would notify our shareholders in advance on our website and by issuing a press release and filing it as additional proxy material with the United States Securities and Exchange Commission (the “SEC”).
What form of identification do I need to be admitted to the meeting?
Via the Internet. Instructions on how to attend and participate via the Internet are posted at www.virtualshareholdermeeting.com/REGN2021. To vote or submit questions during the meeting, you will need the 16-digit control number included on the Notice of Internet Availability of Proxy Materials (the “Notice”) or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received.
In person (if there is in-person attendance option). If you are planningshareholders have the option to attend the meeting, directionsAnnual Meeting in person, the information (including information regarding any procedures and limitations concerning in-person attendees applicable to this location are availablethe Annual Meeting) will be provided in advance on our website and by
issuing a press release and filing it as additional proxy material with the SEC. Please visit our website athttp://newsroom.regeneron.com for the most up-to-date admission requirements for the Annual Meeting.
Can I vote at the Annual Meeting?
Only shareholders of record at the close of business on the record date, April 13, 2021, are entitled to vote at the Annual Meeting. As of April 13, 2021, 104,674,240 shares of the Company’s common stock, par value $0.001 per share (“common stock”), and 1,848,970 shares of Class A stock, par value $0.001 per share (“Class A stock”), were issued and outstanding. The common stock and the Class A stock vote together on all matters as a single class, with the common stock being entitled to one vote per share and the Class A stock being entitled to ten votes per share.
What is on the agenda for the meeting?
| 1 | Election of four Class III directors for a three-year term |
| 2 | Ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2021 |
Can I ask a question at the Annual Meeting?
.Via the Internet. Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be able to submit questions during the meeting, as time permits. If you wish to submit a question during the Annual Meeting, log on to the virtual meeting website using the 16-digit control number, type your question into the “Ask a Question” field, and click “Submit.” Questions and answers may be arranged by topic and substantially similar questions may be answered once.
In person (if there is in-person attendance option). If shareholders have the option to attend the Annual Meeting in person, attendees of the meeting will be given an opportunity to ask questions during a designated question-and-answer period, as time permits.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 3 / 3 |
USERS' GUIDE / GENERAL INFORMATION ABOUT THE MEETING
VOTING INFORMATION
Why did youI receive a notice in the mail regarding the Internet availability of proxy materials instead of a paper copy of the proxy materials?
The "Notice“Notice and Access"Access” rules of the United States Securities and Exchange Commission (the "SEC")SEC permit us to furnish proxy materials, including this proxy statement and our Annual Report on Form 10-K for the fiscal year ended December 31, 20152020 filed with the SEC on February 11, 20168, 2021 (the "2015“2020 Annual Report"Report”), to our shareholders by providing access to such documents on the Internet instead of mailing printed copies. Most shareholders received athe Notice of Internet Availability of Proxy Materials (the "Notice") and will not receive printed copies of the proxy materials unless they request them. This method reduces the environmental impact of the Annual Meeting. The Notice will be mailed beginning on or about April 27, 2016.23, 2021. The Notice includes instructions on how you may access and review all of our proxy materials and the 2020 Annual Report via the Internet. The Notice also includes instructions on how you may vote your shares. If you would like to receive a paper or electronic copy of our proxy materials, you should follow the instructions in the Notice for requesting such materials. Any request to receive proxy materials by mail or e-mail will remain in effect until you revoke it.
Why didn't you receive a notice in the mail about the Internet availability of the proxy materials?
Shareholders who previously elected to access the proxy materials over the Internet will not receive a notice in the mail about the Internet availability of the proxy materials. Instead,
these shareholders should have received an e-mail with links to the proxy materials and the proxy voting website. In addition, shareholders who have previously asked to receive paper copies of the proxy materials and shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan will receive paper copies of the proxy materials.
Can youI vote yourmy shares by filling out and returning the Notice?
No. The Notice identifies the items to be voted on at the Annual Meeting, but you cannot vote by marking the Notice and returning it. The Notice provides instructions on how to vote by Internet, by requesting and returning a paper proxy card, or by submitting a ballot in personvoting at the meeting.
Why did we send youI receive the Notice?
We sent you the Notice regarding this proxy statement because Regeneron'sRegeneron’s board of directors is asking (technically called soliciting) holders of the Company's common stock par value $0.001 per share ("common stock"), and Class A stock par value $0.001 per share ("Class A stock"), to provide proxies to be voted at our 20162021 Annual Meeting of Shareholders or at any adjournment(s) or postponement(s) of the meeting.
Who is entitled to vote at the Annual Meeting?
Only shareholders of record at the close of business on the record date, April 14, 2016, are entitled to vote at the Annual Meeting shares of common stock and/or Class A stock held of record on that date. As of April 14, 2016, 103,165,457 shares of common stock and 1,913,136 shares of Class A stock were issued and outstanding. The common stock and the Class A stock vote together on all matters as a single class, with the common stock being entitled to one vote per share and the Class A stock being entitled to ten votes per share.
6
General Information about the Meeting
What are you being asked to vote on?
We are asking you to vote on:
•election of three Class I directors for a term of three years (Proposal No. 1); and•ratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016 (Proposal No. 2).
What are the board's recommendations?
The board of directors recommends that you vote:
•FORelection of each of the three nominated Class I directors (Proposal No. 1); and•FORratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for 2016 (Proposal No. 2).
How can you vote?
In person. If you are a shareholder of record, you may vote in person at the Annual Meeting. The Company will give you a ballot when you arrive. If you are a beneficial owner of shares held in the name of your bank, broker, or other nominee, or in "street name," to vote in person at the Annual Meeting you must obtain from your nominee and bring to the meeting a "legal proxy" authorizing you to vote such shares held as of the record date. We recommend you vote by proxy even if you plan to attend the meeting. So long as you meet the applicable requirements, you can always change your vote at the meeting. Instructions on voting by proxy are included below.
Via the Internet. You may vote by proxy via the Internet by visitingwww.proxyvote.com. You will need the 12 digit control number included on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received. You may vote via the Internet through 11:59 p.m., Eastern Time, on June 9, 2016.
Via telephone. If you received printed copies of the proxy materials, you may vote by proxy via telephone by calling the toll free number found on the proxy card or the voting instruction form. You will need the 12 digit control number included on the proxy card or voting instruction form. You may vote via telephone through 11:59 p.m., Eastern Time, on June 9, 2016.
By mail. If you received printed copies of the proxy materials, you may vote by proxy by completing the proxy card or voting instruction form and returning it in the envelope provided.
How are proxies voted?
If you vote by proxy in time for it to be voted at the Annual Meeting, one of the individuals named as your proxy will vote your shares as you have directed. If you submit a proxy, but no indication is given as to how to vote your shares as to a proposal, your shares will be voted in the manner recommended by the board of directors. The board of directors knows of no matter, other than those indicated above under "What are you being asked to vote on?"“What is on the agenda for the meeting?”, to be presented at the Annual Meeting. If any other matter properly comes before the Annual Meeting, the persons named and designated as proxies will vote your shares in their discretion.
Why didn’t I receive a notice in the mail about the Internet availability of the proxy materials?
Shareholders who previously elected to receive proxy materials by e-mail will not receive a notice in the mail about the Internet availability of the proxy materials. Instead, these shareholders should have received an e-mail with links to the proxy materials and the proxy voting website. Shareholders who have previously asked to receive paper copies of the proxy materials and shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan will receive paper copies of the proxy materials.
What constitutes a quorum?
The presence at the Annual Meeting, in person or by proxy, of the holders as of the record date of shares of common stock and Class A stock having a majority of the voting power of all shares of common stock and Class A stock outstanding on the record date will constitute a quorum for the transaction of business at the Annual Meeting. Shares held as of the record date by holders who are present or represented by proxy at the Annual Meeting but who have abstained from voting or have not voted with respect to some or all of such shares on any proposal to be voted on at the Annual Meeting will be counted as present for purposes of establishing a quorum.
4 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
USERS' GUIDE / GENERAL INFORMATION ABOUT THE MEETING
7How can I vote my shares without attending the Annual Meeting?
General Information about
We recommend that shareholders vote by proxy even if they plan to attend the Annual Meeting
Table via the Internet or, if applicable, in person. If you are a shareholder of Contentsrecord, there are three ways to vote by proxy:
What
Via the Internet. You may vote is required to approve each proposal?by proxy via the Internet by visiting www.proxyvote.com. You will need the 16-digit control number included on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received. You may vote via the Internet through 11:59 p.m., Eastern Time, on June 10, 2021.
The following table summarizes
Via telephone. You may vote by proxy via telephone by calling the toll-free number found on the proxy card or the voting requirements applicable toinstruction form. You will need the proposals to be voted16-digit control number included on the proxy card or voting instruction form. You may vote via telephone through 11:59 p.m., Eastern Time, on June 10, 2021.
By mail. If you received printed copies of the proxy materials, you may vote by proxy by completing the proxy card or voting instruction form and returning it in the envelope provided.
How can I attend and vote at the Annual Meeting:Meeting?
*As noted above, abstentionsVia the Internet. You may attend the Annual Meeting via the Internet at www.virtualshareholdermeeting.com/REGN2021. Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be
counted as present for purposes of establishing a quorum atable to vote during theAnnual Meeting.
+Only relevant if you are the beneficial owner of shares held in "street name."meeting.In person (if there is in-person attendance option). If you are a shareholder of record and shareholders have the option to attend the Annual Meeting in person, you
do not cast yourmay voteno votes will be cast on your behalf on any of the items of businessin person at the Annual Meeting.
The Company will give you a ballot when you arrive. If you are a beneficial owner of shares held in the name of your bank, broker, or other nominee, or in “street name,” to vote in person at the Annual Meeting you must obtain from your nominee and bring to the meeting a “legal proxy” authorizing you to vote such shares held as of the record date. We recommend you vote by proxy even if you plan to attend the meeting. So long as you meet the applicable requirements, you can always change your vote at the meeting. Instructions on voting by proxy are included above. As also noted above, the Annual Meeting will be a virtual-only meeting unless
the government waiver permitting virtual-only meetings is no longer in effect. Please refer to our website at http://newsroom.regeneron.com for the most up-to-date information.
What if during the Annual Meeting I have technical difficulties or trouble accessing the virtual meeting website?
We will have technicians ready to assist you with any technical difficulties you may have accessing the virtual meeting website. If you encounter any difficulties accessing the virtual meeting website during the meeting time, please call the technical support number that will be posted at www.virtualshareholdermeeting.com/REGN2021.
If I am a Regeneron employee or former employee, how do youI vote shares in the Company Stock Fund in yourmy 401(k) account?account or in the Regeneron Ireland Share Participation Plan?
If you participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan, you may provide voting instructions to Fidelity Management Trust Company, the plan'splan’s trustee, (1) through the Internet atwww.proxyvote.com by 11:59 p.m., Eastern Time, on June 7, 2016,8, 2021, (2) by calling 1-800-690-6903 by 11:59 p.m., Eastern Time, on June 7, 2016,8, 2021, or (3) by returning your completed proxy card by mail. The trustee will vote your shares in accordance with your instructions. If you do not provide timely voting instructions to the trustee, the trustee will vote your shares in the same proportion as the shares for which the trustee receives voting instructions from other participants in the plan.
If you participate and hold shares of common stock in the Regeneron Ireland Share Participation Plan, you may provide voting instructions to Mercer Ireland Limited, who administers the Plan on behalf of Irish Pensions Trust Limited, the trustees of the Plan. You will receive a voting instruction form by mail sent directly to your home address, which you should complete, sign, and return to Mercer by mail using the enclosed pre-paid envelope or as an e-mail attachment in accordance with the instructions provided by Mercer.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 5 / 5 |
USERS' GUIDE / GENERAL INFORMATION ABOUT THE MEETING
Can youI change yourmy vote or revoke yourmy proxy?
Yes. You may change your vote or revoke your proxy at any time before the proxy is exercised. If you votedexercised by proxyvoting again electronically through the Internet or by telephone, as described above,by mailing a new proxy card or voting instruction form, or by attending the Annual Meeting (via the Internet or, if shareholders have this option, in person) and voting. If you are a record holder, you may simply vote again at a later date using the same procedures, in which case the later submitted proxy will be recorded and the earlier vote revoked. If you submittedalso revoke your proxy by mail, you must (i) filefiling with the
Secretary of the Company, at or before the taking of the vote at the Annual Meeting, a written notice of revocation bearing a later date than the proxy you previously submitted or (ii) duly execute a later dated proxy relating to the same shares and deliver it to the Secretary of the Company or other designee before the taking of the vote at the Annual Meeting.submitted. Attendance at the Annual Meeting will not have the effect of revoking a proxy unless you are a record holder and give written notice of revocation to the Secretary of the Company before the proxy is exercised or you vote by written ballot at the Annual Meeting. If you hold your shares through a broker, bank, or other nominee in "street“street name,"” you will need to contact them or follow the instructions in the voting instruction form used by the firm that holds your shares to revoke your proxy. Only your latest dated proxy we receive at or prior to the Annual Meeting will be counted.
Who solicits proxies and bears the cost of solicitation?
Solicitation of proxies may be made by mail, in person, or by telephone by officers, directors, and other employees of the Company and by employees of the Company'sCompany’s transfer agent, American Stock Transfer & Trust Company, LLC ("AST"(“AST”), and employees of Broadridge Financial Solutions, Inc. ("Broadridge"(“Broadridge”). We will reimburse AST, Broadridge, and our banks, brokers, and other custodians, nominees, and fiduciaries for their respective reasonable costs in the preparation and mailing of proxy materials to shareholders. In addition, we
8
General Information about the Meeting
have engaged Innisfree M&A Incorporated to assist in the solicitation of proxies and provide related advice and informational support for a servicesservice fee of $25,000 and the reimbursement of customary disbursements that are not expected to exceed $25,000 in the aggregate.and expenses. We will bear all costs of the solicitation of proxies.
What are the board’s recommendations?
The board of directors recommends that you vote:
 | FORelection of each of the four nominated Class III directors (Proposal No. 1); and |
 | FORratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for 2021 (Proposal No. 2). |
6 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
PleaseUSERS' GUIDE / GENERAL INFORMATION ABOUT THE MEETING
What vote is required to approve each proposal?
The following table summarizes the voting requirements applicable to the proposals to be voted on at the Annual Meeting:
| Proposal | Vote Required | Effect of Abstentions* | Broker Discretionary Voting Allowed?+ | |
| 1 | Election of Directors | Majority of the votes cast. In accordance with our director resignation policy, an incumbent director who fails to receive the required number of votes in an uncontested election will be required to tender his or her resignation to the Chairman of the board of directors for consideration by the Corporate Governance and Compliance Committee. | No effect — not considered votes cast on this proposal | No — brokers without voting instructions will not be able to vote on this proposal |
| 2 | Ratification of the Appointment of PricewaterhouseCoopers LLP | Majority of the votes cast | No effect — not considered votes cast on this proposal | Yes — brokers without voting instructions will have discretionary authority to vote |
| * | As noted above, abstentions will be counted as present for purposes of establishing a quorum at the Annual Meeting. | |
| + | Only relevant if you are the beneficial owner of shares held in “street name.” If you are a shareholder of record and you do not cast your vote, no votes will be cast on your behalf on any of the items of business at the Annual Meeting. |
If any other matter is properly brought before the Annual Meeting, such matter also will be determined by the affirmative vote of a majority of the votes cast at the Annual Meeting.
If shareholders have the option to attend the Annual Meeting in person, please note that cameras, other photographic equipment, or audio or video recording devices will not be permitted at the Annual Meeting.
9
General Information about the Meeting
![]()
Proposal No. 1: Election of Directorsto be used by any in-person attendees.
PursuantINFORMATION ABOUT REGENERON
If you would like to the Company's Certificate of Incorporation, the board of directors is divided into three classes, denominated Class I, Class II, and Class III, with members of each class holding office for staggered three-year terms. There are currently three members in Class I and Class II and four members in Class III. The respective terms of the directors expire (in all cases, subject to the election and qualification of their successors and to their earlier death, resignation, or removal) as follows:
•The terms of the Class I Directors expirelearn more about Regeneron, please visit our website atthe 2016 Annual Meeting;•The terms of the Class II Directors expire at the 2017 Annual Meeting; and•The terms of the Class III Directors expire at the 2018 Annual Meeting.
The board of directors, upon the recommendation of the Corporate Governance and Compliance Committee, has nominated for election at the 2016 Annual Meetingtopics discussed on our website include:
| • | Working at Regeneron |
| • | Our Science Research Mentorship Program |
| • | The Regeneron Science Talent Search |
| • | The Regeneron International Science and Engineering Fair |
| • | The Regeneron DNA Learning Center |
| • | STEM Teaching Fellowship |
| • | Our Graduate Internship Program |
| • | Our Post-doctoral Training Program |
| • | Regeneron employee volunteer programs |
| • | Our patient support programs |
| • | Our approach to corporate responsibility |
| • | Our environmental sustainability efforts |
| • | Our commitment to global transparency |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 7 / 7 |

From Left: N. Anthony Coles, M.D. / Arthur F. Ryan / Michael S.
Brown, M.D., /
George L. Sing / Bonnie L. Bassler, Ph.D. / Leonard S. Schleifer, M.D., Ph.D., and /
P. Roy Vagelos, M.D. / George D. Yancopoulos, M.D., Ph.D. as Class I Directors for a three-year term expiring/ Christine A. Poon /
Joseph L. Goldstein, M.D. / Huda Y. Zoghbi, M.D. / Marc Tessier-Lavigne, Ph.D.
8 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS
As the first substantive order of business at the 20192021 Annual Meeting.
Biographical informationMeeting, you have an opportunity to vote on four members of our board of directors. This is given below, as of April 14, 2016, for each nominee for Class I Director, and for eachthe right starting point not only because the board oversees Regeneron, but because understanding the Regeneron board leads to a better understanding of the otherCompany and its business model.
As our President and CEO has observed, “Our dream when we started Regeneron was to build a company where the scientists would be the heroes.” The composition of Regeneron’s board reflects this founding principle: over half of our directors whose term of office will continue after the 2016 Annual Meeting. All the nominees are presently directors and were previously elected by the shareholders. Nonemembers of the corporationsNational Academy of Sciences, and our board members include two Nobel laureates and holders of many scientific awards. In addition, the board includes individuals with experience building shareholder value through all stages of corporate development. Various members bring substantial governance experience gained from service on other boards and others bring financial, policy, and management expertise. Three of our board’s current 12 members are women, and four directors are diverse by race or other organizations referred to below with which a director has been or is currently employed or otherwise associated is a parent, subsidiary, or affiliateethnicity. Each of the Company.directors who joined the board since 2016 is diverse by gender or race/ethnicity.
The board of directors unanimously recommends a vote FOR the election of Michael S. Brown, M.D., Leonard S. Schleifer, M.D., Ph.D., and George D. Yancopoulos, M.D., Ph.D. as Class I Directors for a three-year term expiring at the 2019 Annual Meeting.
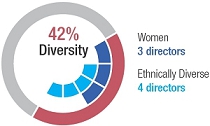
The table below summarizes key qualifications, skills, or attributes most relevant to the decision to nominate the director to serve on the board of directors. A mark indicates a specific area of focus or expertise on which the board of directors relies most. The lack of a mark does not mean the director does not possess that qualification or skill. Each director biography below describes these qualifications and relevant experience in more detail. We believe the table below demonstrates the breadth and diversity of the collective experience, expertise, and skills of our board of directors.
Experience, | Ph.D. | Michael S. Brown, M.D. | N. Anthony Coles, M.D. | Joseph L. Goldstein, M.D. | Christine A. Poon | Arthur F. Ryan | Leonard S. Schleifer, M.D., Ph.D. | George L. Sing | Marc Tessier- Lavigne, Ph.D. | P. Roy Vagelos, M.D. | George D. Yancopoulos, M.D., Ph.D. | ||||||||||||||
| Zoghbi, M.D. | ||||||||||||||||||||||||
| Industry Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Executive/Leadership Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Science/Biotech Background | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Research/Academic Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Business Strategy/ Operations Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Financial Expertise | ● | ● | ● | ● | ● | ● | |||||||||||||||||||
| Public Company CEO Experience | |||||||||||||||||||||||||
National Academy of Sciences Membership | |||||||||||||||||||||||||
10
Proposal No. 1: Election of Directors
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
|
BOARD OF DIRECTORS / MEET THE BOARD
NOMINEES FOR CLASS III DIRECTORS
FOR ELECTION AT THE 2021 ANNUAL MEETING FOR A TERM EXPIRING AT THE 2024 ANNUAL MEETING1
N. ANTHONY COLES, M.D.

Director since: 2017
Age: 60
Independent
Other Public Boards:
| • | Cerevel Therapeutics Holdings, Inc. |
| • | Yumanity Therapeutics, Inc. |
| • | McKesson Corporation* |
| * | Dr. Coles has notified McKesson Corporation that he will not stand for reelection as a member of its board of directors at the end of his current term, which will expire at McKesson’s 2021 annual meeting of shareholders. |
Experience and Qualifications
Dr. Coles serves as the President, Chief Executive Officer, and Chairperson of Cerevel Therapeutics Holdings, Inc., the parent entity of Cerevel Therapeutics, Inc., a biopharmaceutical company specializing in the development of new therapies for diseases of the central nervous system. Previously, from 2014 to 2019, Dr. Coles served as Chief Executive Officer of Yumanity Therapeutics, LLC (now known as Yumanity Therapeutics, Inc. ("Yumanity")), a company focused on transforming drug discovery for neurodegenerative diseases, and continues to serve as the Executive Chairman of the board of directors of Yumanity. From 2013 to present, Dr. Coles has served as Chairman and CEO of TRATE Enterprises LLC, a privately-held company. Dr. Coles served as President, Chief Executive Officer and Chairman of the Board of Onyx Pharmaceuticals, Inc., a biopharmaceutical company, from 2012 until 2013, having served as its President, Chief Executive Officer, and a member of its board of directors from 2008 until 2012. Prior to joining Onyx in 2008, he was President, Chief Executive Officer, and a member of the board of directors of NPS Pharmaceuticals, Inc., a biopharmaceutical company. Before joining NPS in 2005, he served in various leadership positions in the biopharmaceutical and pharmaceutical industries, including at Merck & Co., Inc., Bristol-Myers Squibb Company, and Vertex Pharmaceuticals Incorporated. In addition to having previously served as a director of Onyx and NPS, he was formerly a director of Laboratory Corporation of America Holdings, Campus Crest Communities, Inc., and CRISPR Therapeutics AG.
Dr. Coles has been a director of McKesson Corporation since April 2014 and serves on the Compensation Committee and the Finance Committee of its board of directors.
The experience of Dr. Coles as a seasoned executive and corporate director with extensive knowledge of highly regulated biopharmaceutical and pharmaceutical companies, as well as his in-depth knowledge and understanding of the regulatory environment in which Regeneron operates, led to the board's decision to nominate Dr. Coles for reelection to the board.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 9/11 |
| Audit Committee: |
Prior Voting Results—2020:
| For: | 99.5% |
| Against: | 0.5% |
Regeneron Common Stock Beneficially Owned as of April 13, 2021:
| Common Stock: | 11 |
| Options: | 29,657 |
| Restricted Stock Units (“RSUs”): | 750 |
| 1 | Biographical information is given, as of April 13, 2021, for each nominee and for each of the other directors whose term of office will continue after the 2021 Annual Meeting. All the nominees are presently directors and were previously elected by the shareholders. None of the corporations or other organizations referred to below with which a director has been or is currently employed or otherwise associated is a parent, subsidiary, or affiliate of the Company. |
  |
BOARD OF DIRECTORS / MEET THE BOARD
ARTHUR F. RYAN

Director since: 2003
Age: 78
Independent
Experience and Qualifications
In 2008, Mr. Ryan retired as the Chairman of the Board of Prudential Financial, Inc., one of the largest diversified financial institutions in the world. He served as Chief Executive Officer of Prudential until 2007. Prior to joining Prudential in 1994, Mr. Ryan served as President and Chief Operating Officer of Chase Manhattan Bank since 1990. Mr. Ryan managed Chase's worldwide retail bank between 1984 and 1990. From 2008 to 2013, Mr. Ryan served as a non-executive director of the Royal Bank of Scotland Group plc. From 2009 to 2019, Mr. Ryan served as a director of Citizens Financial Group, Inc., a retail bank holding company that became publicly traded in 2014, and also served as its lead director, chair of the Compensation and Human Resources Committee, and a member of the Nominating and Corporate Governance Committee.
Mr. Ryan's substantial leadership experience as a chief executive officer of leading companies in the banking and insurance industries, and his extensive business experience and financial expertise, led to the board's decision to nominate Mr. Ryan for reelection to the board.
Board and Committee Membership—2020 Attendance:
| Board of | 10/11 | |||
Audit Committee: | 11/11 | |||
| Corporate Governance and Compliance Committee (Chairman): |
Prior Voting Results—2018:
| For: | 88.8% |
| Against: | 11.2% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 22,800 |
| Options: | 8,404 |
| RSUs: | 750 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 11 / 11 |
BOARD OF DIRECTORS / MEET THE BOARD
GEORGE L. SING

Director since: 1988
Age: 71
Independent
Experience and Qualifications
Since 1998, Mr. Sing has been a Managing Director of Lancet Capital, a venture capital investment firm in the healthcare field. In addition, since 2016, Mr. Sing has served as Chief Executive Officer of GanD, Inc., a biomedical drug development company.
Mr. Sing's extensive healthcare and financial expertise as a healthcare venture capital investor and biomedical company chief executive officer, his executive leadership experience, and his substantial knowledge of the Company led to the board's decision to nominate Mr. Sing for reelection to the board.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 11/11 |
| Audit Committee (Chairman): | 11/11 |
| Compensation Committee: | 13/13 |
Prior Voting Results—2018:
| For: | 64.0% |
| Against: | 36.0% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 59,022 |
| Options: | 45,028 |
| RSUs: | 750 |
  |
BOARD OF DIRECTORS / MEET THE BOARD
MARC TESSIER-LAVIGNE, PH.D.

Director since: 2011
Age: 61
Independent
Scientific Society Memberships:
| • | The National Academy of |
| • | The National Academy of Medicine |
| • | The Royal Society of London |
| • | The Royal Society of Canada |
Other Public Boards:
Experience and Qualifications
Dr. Tessier-Lavigne has been the President of Stanford University since 2016. Before assuming his role at Stanford, he served as the President of The Rockefeller University and a Carson Family Professor and head of the Laboratory of Brain Development at The Rockefeller University from 2011. Previously, he served as Executive Vice President and Chief Scientific Officer at Genentech, Inc., which he joined in 2003. He was a professor at Stanford University from 2001 to 2003 and at the University of California, San Francisco from 1991 to 2001. Dr. Tessier-Lavigne is a member of the National Academy of Sciences, the National Academy of Medicine, and a fellow of the Royal Societies of London and Canada. Dr. Tessier-Lavigne is a member of the Board of Directors of Denali Therapeutics Inc., and previously served on the board of directors of Pfizer Inc., Agios Pharmaceuticals, Inc., and Juno Therapeutics, Inc.
Dr. Tessier-Lavigne's distinguished scientific and academic background, and his significant industry experience, including experience in senior scientific leadership roles at a leading biopharmaceutical company, led to the board's decision to nominate Dr. Tessier-Lavigne for reelection to the board.
Board and Committee Membership—2020 Attendance:
| Board of | 7/11 |
Technology Committee: | 3/3 |
Prior Voting Results—2018:
| For: | 94.8% | ||
| Against: |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 1,187 |
| Options: | 57,778 |
| RSUs: | 750 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 13 / 13 |
BOARD OF DIRECTORS / MEET THE BOARD
CLASS I DIRECTORS CONTINUING IN OFFICE
TERM EXPIRES AT THE 2022 ANNUAL MEETING
BONNIE L. BASSLER, PH.D.

Director since: 2016
Age: 58
Independent
Scientific Society Memberships:
| • | The National Academy of Sciences |
| • | The American Academy of Arts and Sciences |
| • | The Royal Society of London |
| • | The American Philosophical Society |
Other Public Boards:
| • | Kaleido Biosciences, Inc. |
| • | Cidara Therapeutics, Inc. |
| • | Royalty Pharma plc |
Experience and Qualifications
Dr. Bassler is currently the Chair of the Department of Molecular Biology and the Squibb Professor in Molecular Biology at Princeton University, and a Howard Hughes Medical Institute Investigator. Dr. Bassler has previously served as the President of the American Society for Microbiology, as well as on the boards for the American Association for the Advancement of Science, the National Science Foundation, and the American Academy of Microbiology. She has been elected to the National Academy of Sciences, the American Academy of Arts and Sciences, the Royal Society of London, and the American Philosophical Society, and has received many scientific honors, including a MacArthur Foundation Fellowship, the Lounsbery Award, and the Shaw Prize for Life Science and Medicine. Dr. Bassler received her B.Sc. from the University of California, Davis, and her Ph.D. in Biochemistry from Johns Hopkins University. She served as a Postdoctoral Fellow and Research Scientist at the Agouron Institute in La Jolla, California, before becoming a faculty member at Princeton University. Dr. Bassler served as a director of Sanofi from November 2014 to July 2016 and currently serves on the board of directors of Kaleido Biosciences, Inc., Cidara Therapeutics, Inc., and Royalty Pharma plc.
Dr. Bassler's extensive research experience and her scientific and academic career and accomplishments, as well as her experience as a corporate director, led the board to conclude that Dr. Bassler should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 11/11 |
| Corporate Governance and Compliance Committee: | 5/5 |
| Technology Committee: | 3/3 |
Prior Voting Results—2019:
| For: | 75.4% |
| Against: | 24.6% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 0 |
| Options: | 29,821 |
| RSUs: | 750 |
  |
BOARD OF DIRECTORS / MEET THE BOARD
MICHAEL S. BROWN, M.D.

Director since: 1991
Age: 80
Independent
Scientific Society Memberships:
| • | The National Academy of | ||
| • | The National Academy of | ||
| • | The Royal Society of | London |
Experience and Qualifications
Dr. Brown holds the Distinguished Chair in Biomedical Sciences, a position he has held since 1989, and is a Regental Professor of Molecular Genetics and Internal Medicine, and the Director of the Jonsson Center for Molecular Genetics, at The University of Texas Southwestern Medical Center at Dallas, positions he has held since 1985. Drs. Brown and Goldstein jointly received the Nobel Prize for Physiology or Medicine in 1985 and the U.S. National Medal of Science in 1988. Dr. Brown is a member of the National Academy of Sciences, the National Academy of Medicine, and Foreign Member of the Royal Society of London.
Dr. Brown's distinguished scientific and academic background, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his significant industry experience gained through his service on the board of directors of the Company and of a leading pharmaceutical company, led the board to conclude that Dr. Brown should serve as a director.
11Board and Committee Membership—2020 Attendance:
Proposal No. 1: Election of Directors
|
| Board of Directors: | 11/11 | |||
| Corporate Governance and Compliance Committee: | ||||
| Technology Committee (Chairman): | 3/3 |
Prior Voting Results—2019:
| For: | 70.7% |
| Against: | 29.3% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 15,349 |
| Options: | 28,625 |
| RSUs: | 750 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 15 / 15 |
BOARD OF DIRECTORS / MEET THE BOARD
LEONARD S. SCHLEIFER, M.D., PH.D.

Director since: 1998
Age: 68
Experience and Qualifications
Dr. Schleifer founded the Company in 1988, has been a director and its President and Chief Executive Officer since its inception, and served as Chairman of the Board from 1990 through 1994. Dr. Schleifer, together with Regeneron's founding scientist, Dr. Yancopoulos, built and has managed the Company over the past 33 years. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology. With over 30 years of experience as Chief Executive Officer of the Company, Dr. Schleifer brings to the board an incomparable knowledge of the Company, significant leadership experience, and an in-depth understanding of the complex research, drug development, and business issues facing companies in the biopharmaceutical industry.
Dr. Schleifer's significant industry and leadership experience, as well as his extensive knowledge of the Company, led the board to conclude that Dr. Schleifer should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 10/11 |
| Technology Committee: | 3/3 |
Prior Voting Results—2019:
| For: | 83.8% |
| Against: | 16.2% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Class A Stock: | 1,726,565 |
| Common Stock: | 580,347 |
| Options: | 1,632,488 |
  |
BOARD OF DIRECTORS / MEET THE BOARD
GEORGE D. YANCOPOULOS, M.D., PH.D.

Director since: 2001
Age: 61
Scientific Society Memberships:
Experience and Qualifications
Dr. Yancopoulos joined Dr. Schleifer in 1989 as founding scientist of the Company, and together they built and have managed the Company since then. Dr. Yancopoulos is currently President and Chief Scientific Officer, and has served on the board since 2001.
He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and/or developer of the nine FDA-approved drugs the Company has developed, EYLEA® (aflibercept) Injection, Praluent® (alirocumab), Dupixent® (dupilumab), Kevzara® (sarilumab), Libtayo® (cemiplimab), EvkeezaTM (evinacumab-dgnb), Inmazeb® (atoltivimab, maftivimab and odesivimab-ebgn), ZALTRAP® (ziv-aflibercept) Injection for Intravenous Infusion, and ARCALYST® (rilonacept) Injection for Subcutaneous Use, as well as of its foundation technologies, including the TRAP technology, VelociGene®, and VelocImmune®. As one of the few members of the National Academy of Sciences from industry and as an author of a substantial number of scientific publications, Dr. Yancopoulos has a distinguished record of scientific expertise. Dr. Yancopoulos also brings to the board his experience in building and managing the Company, his in-depth knowledge of the Company's technologies and research and development programs, and his proven track-record for envisioning successful long-term strategic directions and opportunities.
Dr. Yancopoulos's significant industry and scientific experience, as well as his extensive knowledge of the Company, led the board to conclude that Dr. Yancopoulos should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of | 9/11 |
Technology Committee: | 3/3 |
Prior Voting Results—2019:
| For: | 83.7% | ||
| Against: |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Class A Stock: | 42,750 |
| Common Stock: | 1,286,997 |
| Options: | 1,098,053 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 17 / 17 |
BOARD OF DIRECTORS / MEET THE BOARD
CLASS II DIRECTORS CONTINUING IN OFFICE
TERM EXPIRES AT THE 2023 ANNUAL MEETING
JOSEPH L. GOLDSTEIN, M.D.

Director since: 1991
Age: 80
Independent
Scientific Society Memberships:
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The Royal Society of London |
Experience and Qualifications
Dr. Goldstein has been a Professor of Molecular Genetics and Internal Medicine and the Chairman of the Department of Molecular Genetics at The University of Texas Southwestern Medical Center at Dallas since 1977. Dr. Goldstein is a member of the National Academy of Sciences, the National Academy of Medicine, and the Royal Society of London. He also serves on the Boards of Trustees of The Rockefeller University and the Howard Hughes Medical Institute. Drs. Goldstein and Brown jointly received the Nobel Prize for Physiology or Medicine in 1985 and the U.S. National Medal of Science in 1988.
Dr. Goldstein's extensive research experience, his distinguished scientific and academic credentials, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his substantial understanding of the Company gained through his service as a director since 1991, led the board to conclude that Dr. Goldstein should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 11/11 |
| Corporate Governance and Compliance Committee: | 5/5 |
| Technology Committee: | 3/3 |
Prior Voting Results—2020:
| For: | 95.1% |
| Against: | 4.9% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 5,000 |
| Options: | 8,404 |
| RSUs: | 750 |
  |
BOARD OF DIRECTORS / MEET THE BOARD
CHRISTINE A. POON

Director since: 2010
Age: 68
Other Public Boards:
| • | Prudential Financial, Inc. |
| • | The Sherwin-Williams Company |
| • | Decibel Therapeutics, Inc.* |
| • | Royal Philips Electronics* |
| * | Ms. Poon is a director of |
Experience and Qualifications
Ms. Poon is an Executive-in-Residence in the Department of Management and Human Resources at The Max M. Fisher College of Business at The Ohio State University, where she served as Dean and the John W. Berry, Sr. Chair in Business from 2009 to 2014. Prior to joining Fisher, Ms. Poon spent eight years at Johnson & Johnson, most recently as vice chairman and worldwide chairman of pharmaceuticals. At Johnson & Johnson, she served on the company's board of directors and executive committee and was responsible for managing the pharmaceutical businesses of the company. Prior to joining Johnson & Johnson, Ms. Poon spent 15 years at Bristol-Myers Squibb Company, a global pharmaceutical company, where she held senior leadership positions including president of international medicines and president of medical devices. Ms. Poon serves on the boards of directors of Prudential Financial, Inc., The Sherwin-Williams Company, and Decibel Therapeutics, Inc. and the Supervisory Board of Royal Philips Electronics.
Ms. Poon's extensive expertise in domestic and international business operations, including sales and marketing and commercial operations, and her deep strategic and operational knowledge of the pharmaceutical industry, led the board to conclude that Ms. Poon should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of | 11/11 | |||
Compensation Committee (Chairperson): | 13/13 | |||
| Corporate Governance and Compliance Committee: |
Prior Voting Results—2020:
| For: | 91.5% |
| Against: | 8.5% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 790 |
| Options: | 87,308 |
| RSUs: | 750 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 19 / 19 |
BOARD OF DIRECTORS / MEET THE BOARD
P. ROY VAGELOS, M.D.

Director since: 1995
Age: 91
Scientific Society Memberships:
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The American Philosophical Society |
Experience and Qualifications
Prior to joining Regeneron, Dr. Vagelos was Chairman of the Board and Chief Executive Officer of Merck & Co., Inc., a global pharmaceutical company. He joined Merck in 1975, became a director in 1984, President and Chief Executive Officer in 1985, and Chairman in 1986. Dr. Vagelos retired from all positions with Merck in 1994. Dr. Vagelos is a member of the National Academy of Sciences, the National Academy of Medicine, and the American Philosophical Society. During his tenure as Chairman of Regeneron and previously as Chairman and Chief Executive Officer of Merck, Dr. Vagelos developed an extensive understanding of the complex business, operational, scientific, regulatory, and commercial issues facing the pharmaceutical industry.
Dr. Vagelos's tenure and experience with the Company and Merck, his extensive knowledge of the pharmaceutical industry, his substantial leadership experience, and his significant understanding of the Company led the board to conclude that Dr. Vagelos should serve as a director.
Board and Committee Membership—2020 Attendance:
| Board of Directors: | 11/11 |
| Technology Committee: | 3/3 |
Prior Voting Results—2020:
| For: | 98.4% |
| Against: | 1.6% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 636,427 |
| Options: | 637,530 |
  |
BOARD OF DIRECTORS / MEET THE BOARD
HUDA Y. ZOGHBI, M.D.

Director since: 2016
Age: 66
Independent
Scientific Society Memberships:
| • | The National Academy of | ||
| • | The Institute of Medicine | ||
| • | The American Association for the | Science |
Experience and Qualifications
Dr. Zoghbi is currently a professor in the departments of Pediatrics, Molecular and Human Genetics, and Neurology and Neuroscience at Baylor College of Medicine, the director of the Jan and Dan Duncan Neurological Research Institute at Texas Children's Hospital, and an investigator of the Howard Hughes Medical Institute. She has been elected to the National Academy of Sciences, the Institute of Medicine, and the American Association for the Advancement of Science, and has been awarded numerous recognitions for her work, including the Pearl Meister Greengard Prize, the March of Dimes Prize in Developmental Biology, and the Vanderbilt Prize in Biomedical Science.
Dr. Zoghbi earned her B.Sc. from the American University of Beirut, received her M.D. from Meharry Medical College in Nashville, Tennessee, and completed her pediatrics residency and a joint residency in neurology and pediatric neurology at Baylor College of Medicine, where she then pursued postdoctoral research training in molecular genetics.
Dr. Zoghbi’s extensive research experience and her scientific and academic career and accomplishments led the board to conclude that Dr. Zoghbi should serve as a director.
12Board and Committee Membership—2020 Attendance:
Proposal No. 1: Election of Directors
|
| Board of Directors: | 9/11 | |||
| Compensation Committee: | ||||
| Technology Committee: |  |
Prior Voting Results—2020:
| For: | |
| 4.4% |
Regeneron Securities Beneficially Owned as of April 13, 2021:
| Common Stock: | 0 |
| Options: | 24,703 |
| RSUs: | 750 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 21 / 21 |
The board has a standing Audit Committee, Compensation Committee, and Corporate Governance and Compliance Committee, each of which is comprised entirely of independent directors. The Corporate Governance and Compliance Committee is responsible for reviewing and recommending for the board’s selection candidates to serve on our board of directors and for overseeing all aspects of the Company’s compliance program other than financial compliance. The board also has a standing Technology Committee. The board has adopted charters for the Audit Committee, Compensation Committee, Corporate Governance and Compliance Committee, and Technology Committee, current copies of which are available on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page.
We show below information on the membership, key functions, and number of meetings of each board committee during 2020.
| AUDIT COMMITTEE |
| Members | Key Functions |
George L. Sing, Chairman Number of Meetings Held in 2020 11 | • Select the independent registered public accounting firm, review and approve its engagement letter, and monitor its independence and performance. • Review the overall scope and plans for the annual audit by the independent registered public accounting firm. • Approve performance of non-audit services by the independent registered public accounting firm and evaluate the performance and independence of the independent registered public accounting firm. • Review and approve the Company’s periodic financial statements and the results of the year-end audit. • Review and discuss the adequacy and effectiveness of the Company’s accounting and internal control policies and procedures. • Evaluate the internal audit process for establishing the annual audit plan; review and approve the appointment and replacement of the Company’s Chief Audit Executive, if applicable, and any outside entities providing internal audit services and evaluate their performance on an annual basis. • Review the independent registered public accounting firm’s recommendations concerning the Company’s financial practices and procedures. • Oversee the Company’s risk management program. • Discuss with management the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures. • Establish procedures for the receipt, retention, and treatment of complaints received by the Company • Review and approve any related person transaction. • Prepare an annual report of the Audit Committee for inclusion in the Company’s proxy statement. |
22 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / BOARD COMMITTEES |
| COMPENSATION COMMITTEE |
| Members | Key Functions |
Christine A. Poon, Chairperson Number of Meetings Held in 2020 13 | • Evaluate the performance of the Chief Executive Officer and other executive officers of • Recommend compensation for the Chief Executive Officer for approval by the non-employee members of the board of • Approve compensation for other executive officers. • Approve the total compensation budget for all Company employees. • Oversee the Company’s compensation and • Review and approve annually the • Review and approve the Compensation Discussion and Analysis to be included in the Company’s proxy statement. • Prepare an annual report of the Compensation Committee for inclusion in the Company’s proxy statement. |
| CORPORATE GOVERNANCE AND COMPLIANCE COMMITTEE |
| Members | Key Functions |
Arthur F. Ryan, Chairman Number of Meetings Held in 2020 5 | • Identify qualified individuals to become members of the board and recommend such candidates to • Assess the functioning of the board and its committees and make recommendations to the board concerning the appropriate size, function, and needs of the board. • Review, and make recommendations to the board regarding, non-employee director compensation. • Make recommendations to the board regarding corporate governance matters and practices. • Oversee all aspects of the Company’s comprehensive compliance program other than financial compliance. • Oversee the Company’s key corporate responsibility initiatives and conduct a |
| TECHNOLOGY COMMITTEE |
| Members | Key Functions |
Michael S. Brown, M.D., Chairman Number of Meetings Held in 2020 3 | • Review and evaluate the Company’s research and clinical development programs, plans, and policies. |
+ Ex Officio Member.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 23 / 23 |
13
Proposal No. 1: Election of Directors
 | |||||
TableIncorporation, the board of Contents
![]()
Corporate Governancedirectors is divided into three classes, denominated Class I, Class II, and Class III, with members of each class holding office for staggered three-year terms. There are currently four members in each of Class I, Class II, and Class III. The respective terms of the directors expire (in all cases, subject to the election and qualification of their successors and to their earlier death, resignation, or removal) as follows:
OverviewBOARD MEETINGS AND ATTENDANCE OF DIRECTORS
The board held 11 meetings in 2020, of which five were regular and six were special meetings. The Regeneron is committedBoard of Directors Corporate Governance Guidelines provide that directors are expected to good corporate governance, which we believe promotes the long-term interests of shareholders, strengthens the accountabilityattend all or substantially all meetings of the board and the committees on which they serve. All directors attended at least 75% of directors
the total number of meetings of the board and management,committees of the board on which they served, except for Dr. Tessier-Lavigne, who, while attending all of the regularly scheduled board and helps build trustapplicable committee meetings, was unable to attend certain special board meetings called on short notice due in large part to the fact that these meetings had been scheduled without sufficient regard for time zone differences, resulting in his attendance falling below 75% by one meeting. In 2021 to date, Dr. Tessier-Lavigne has attended 100% of the board and applicable committee meetings.
In considering whether to recommend Dr. Tessier-Lavigne for reelection to the board at the 2021 Annual Meeting of Shareholders, the Corporate Governance and Compliance Committee took into account that Dr. Tessier-Lavigne attended all of the regularly scheduled board and applicable committee meetings in 2020 and 100% of the board and applicable committee meetings in 2021 to date; that a number of the 2020 meetings that he was unable to attend were called on short notice, without sufficient regard to the fact that Dr. Tessier-Lavigne was the only director based in the Company. The following chart summarizes key information regardingPacific Time Zone; that appropriate steps had been taken to ensure that all future meetings would be scheduled so as not to disadvantage Dr. Tessier-Lavigne because of his location; and that Dr. Tessier-Lavigne has a long history of good meeting attendance. In light of these factors, the Corporate Governance and Compliance Committee believes that Dr. Tessier-Lavigne’s attendance will not be a future concern and, having taken into consideration his distinguished scientific and academic career and his significant industry experience, recommended to the board that Dr. Tessier-Lavigne should be nominated for reelection to the board.
According to the Regeneron Board of Directors Corporate Governance Guidelines, board members are expected to attend the Company’s Annual Meeting of Shareholders. All of the directors attended our corporate governance.
| ||
| ||
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
2020 Annual |
| |
|
| |
|
| |
|
| |
|
| |
*AsofApril 14, 2016.
Procedures Relating to NomineesPROCEDURES RELATING TO NOMINEES; BOARD SUCCESSION PLANNING
The Corporate Governance and Compliance Committee will consider a nominee for election to the board of directors recommended by a shareholder of record if the shareholder submits the nominationrecommendation in compliance with the requirements of our by-laws and the Guidelines Regarding Director Nominations, which are available on our website atwww.regeneron.comunder the "Corporate Governance"“Corporate Governance” heading on the "Investors“Investors & Media"Media” page.
In considering potential candidates for the board of directors, the Corporate Governance and Compliance Committee considers factors such as whether or not a potential candidate: (1) possesses relevant expertise; (2) brings skills and experience complementary to those of the other members of the board; (3) has sufficient time to devote to the affairs of the Company; (4) has demonstrated excellence in his or her field; (5) has the ability to exercise sound business
24 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / BOARD GOVERNANCE |
judgment; (6) has the commitment to rigorously represent the long-term interests of the Company'sCompany’s shareholders; (7) possesses a diverse background and experience, including with respect to race, age, and gender; and (8) such other factors as the Corporate Governance and Compliance Committee may determine from time to time.
Candidates for director are reviewed in the context of the current composition of the board of directors, the operating requirements of the Company, and the long-term interests of
shareholders. In conducting the assessment, the Committee considers the individual'sindividual’s independence, experience, skills, background, and diversity, including with respect to race, age, and gender, along with such other factors as it deems appropriate, given the current needs of the board and the Company to maintain a balance of knowledge, experience, and capabilities. When recommending a slate of director nominees each year, the Corporate Governance and Compliance Committee reviews the current composition of the board of directors in order to recommend a slate of directors who, with the continuing directors, will provide the board with the requisite diversity of skills, expertise, experience, and viewpoints necessary to effectively fulfill its duties and responsibilities.
In the case of an incumbent director whose term of office is set to expire, the Corporate Governance and Compliance Committee reviews such director'sdirector’s overall service to the Company during the director'sdirector’s term and also considers the director'sdirector’s interest in continuing as a member of the board. In the case of a new director candidate, the Corporate Governance and Compliance Committee also reviews whether the nominee is "independent,"“independent,” based on our Corporate Governance Guidelines, applicable listing standards of the NASDAQNasdaq Stock Market LLC, and applicable SEC and other relevant rules and regulations, if necessary.
The Corporate Governance and Compliance Committee may employ a variety of methods for identifying and evaluating
15
Corporate Governance
nominees for the board of directors. TheIn addition, the Corporate Governance and Compliance Committee may consider candidates recommended by other directors, management, search firms, shareholders, or other sources. When conducting searches for new directors, the Corporate Governance and Compliance Committee will take reasonable steps to include diverse candidates in the pool of nominees and any search firm will affirmatively be instructed to seek to include diverse candidates. Candidates recommended by shareholders will be evaluated on the same basis as candidates recommended by our directors or management or by third partythird-party search firms or other sources. Candidates may be evaluated at regular or special meetings of the Corporate Governance and Compliance Committee.
The Corporate Governance and Compliance Committee seeks to Remove Directorsensure that our board of directors as a whole possesses the mix of skills and experiences to provide effective oversight and guidance to management to execute on the Company’s long-term strategy. The Committee also considers succession planning for Causeboard and committee chairs for purposes of continuity and to Call Special Shareholder Meetingmaintain relevant expertise and depth of experience.
Regeneron's

BOARD AND COMMITTEE SELF-ASSESSMENTS
On an annual basis, the board of directors, the Audit Committee, the Compensation Committee, and the Corporate Governance and Compliance Committee conduct self-assessments to ensure effective performance and to identify opportunities for improvement. As the first step in the self-assessment process, directors complete a comprehensive questionnaire, which asks them to consider various topics related to board and committee composition, structure, effectiveness, and responsibilities, as well as satisfaction with the schedule, materials, and discussion topics. Each committee, as well as the board as a whole, then reviews and assesses the responses and presents its findings and recommendations to the board of directors. The results of the assessments are then discussed by the board of
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 25 / 25 |
| BOARD OF DIRECTORS / BOARD GOVERNANCE |
directors and the respective committees in executive session, with a view toward taking action to address any issues presented. Results requiring additional consideration are addressed at subsequent board and committee meetings, where appropriate.
Annual Self-Assessment Process
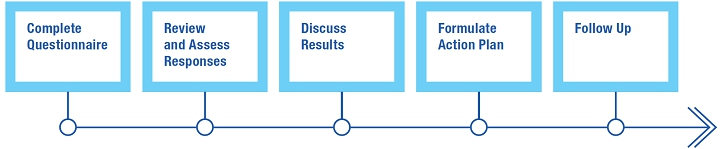
While this formal self-assessment is conducted on an annual basis, directors share perspectives, feedback, and suggestions year-round, both inside and outside the boardroom.
SHAREHOLDER RIGHTS TO REMOVE DIRECTORS FOR CAUSE AND TO CALL SPECIAL SHAREHOLDER MEETING
Regeneron’s charter documents give shareholders the rights to (i) remove directors for cause by an affirmative vote of at least 80% of the outstanding shares of all classes of capital stock entitled to vote for directors; and (ii) call a special shareholder meeting upon the written request of at least 25% of the total number of votes entitled to be cast by shareholders.
Shareholder Communications with DirectorsDIRECTOR INDEPENDENCE
The Company has established a process for shareholders to send communications to the members of the board of directors. Shareholders may send such communications by mail addressed to the full board, a specific member or members of the board, or a particular committee of the board, at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707,
Attention: Corporate Secretary. All such communications will be opened by our Corporate Secretary for the sole purpose of determining whether the contents represent a message to our directors. Any contents that are not in the nature of advertising, promotions of a product or service, or patently offensive material will be forwarded promptly to the addressee. In the case of communications to the board or any individual director or group or committee of directors, the Corporate Secretary will make sufficient copies of the contents to send to such director or each director who is a member of the group or committee to which the envelope is addressed.
The board has a standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), a Compensation Committee, and a Corporate Governance and Compliance Committee, each of which is comprised entirely of independent directors. The Corporate Governance and Compliance Committee is responsible for reviewing and recommending for the board's selection candidates to serve on our board of directors and for overseeing all aspects of the Company's compliance program other than financial compliance. The board also has a standing Technology Committee. The board has adopted charters for the Audit Committee, Compensation Committee, Corporate Governance and Compliance Committee, and Technology Committee, current copies of which are available on our website atwww.regeneron.com under the "Corporate Governance" heading on the "Investors & Media" page.
16
Corporate Governance
We show below information on the membership, key functions, and number of meetings of each board committee during 2015.
|
|
17
Corporate Governance
|
| |||||
| ||||||
|
| |||||
|
| |||||
+Dr. Tessier-Lavigne resigned as Chairman and a member of the Compensation Committee effective as of April 1, 2016. Ms. Poon was appointed as Chairperson of the Compensation Committee effective as of Dr. Tessier-Lavigne's resignation date.
*Ex OfficioMember.
The board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers, and directors. You can find links to this code on our website atwww.regeneron.com under the "Corporate Governance" heading on the "Investors & Media" page. We may satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding
an amendment to, or a waiver from, a provision of our code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or controller, or persons performing similar functions, by posting such information on our website where it is accessible through the same link noted above.
18
Corporate Governance
The board of directors has determined that each of the following currently serving directors is independent as defined in the listing standards of The NASDAQNasdaq Stock Market LLC and our Corporate Governance Guidelines: Charles A. Baker,Bonnie L. Bassler, Ph.D., Michael S. Brown, M.D., N. Anthony Coles, M.D., Joseph L. Goldstein, M.D., Christine A. Poon, Arthur F. Ryan, George L. Sing, and Marc Tessier-Lavigne, Ph.D., and Huda Y. Zoghbi, M.D. These individuals are affiliated with numerous educational institutions, hospitals, charities, and corporations, as well as civic organizations and professional associations. The board of directors considered each of these relationships and determined that none of these relationships conflicted with the interests of the Company or would impair their independence or judgment. TheIn accordance with our Corporate Governance Guidelines, the board conducts executive sessions of independent directors presided by the Chairman of the Corporate Governance and Compliance Committee following each regularly scheduled board meeting.
The board of directors has determined that each of the current members of the Audit Committee, Messrs. Baker, Ryan and Sing and Dr. Coles, qualifies as an "audit“audit committee financial expert"expert” as that term is defined in Item 407(d)(5)(ii) of Regulation S-K under the Exchange Act, meets the required standards for independence set forth in Rule 10A-3(b)(1) under the Exchange Act,by SEC rules, and is independent as defined for audit committee members in the listing standards of The NASDAQNasdaq Stock Market LLC.LLC and SEC rules.
In addition, the board of directors has determined that each of the current members of the Compensation Committee, Ms. Poon, Messrs. Baker andMr. Sing, and Dr. Goldstein,Zoghbi, meets the additional independence criteria applicable to compensation committee members under the listing standards of The NASDAQNasdaq Stock Market LLC and qualifies as a "Non-Employee Director"“Non-Employee Director” pursuant to Rule 16b-3 under the Securities Exchange Act andof 1934, as an "outside director" within the meaning of Section 162(m) of the Internal Revenue Code.amended (the “Exchange Act”).
26 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / BOARD GOVERNANCE |
Board Leadership and Role in Risk OversightBOARD LEADERSHIP AND ROLE IN RISK OVERSIGHT
The board of directors recognizes that one of its key responsibilities is to establish and evaluate an appropriate leadership structure for the board so as to provide effective oversight of management. Since 1995, the board has separated the roles of the Chief Executive Officer and the Chairman of the Board, with Dr. Vagelos serving as Chairman and Dr. Schleifer serving as President and Chief Executive Officer. Dr. Vagelos's extensive leadership experience, his business acumen, and his deep understanding of the healthcare industry have made him an invaluable resource to both the board and Dr. Schleifer. The board has determined that this leadership structure is appropriate for the Company at this time. This determination was based in part on Dr. Vagelos’s extensive leadership experience, business acumen, and deep understanding of the healthcare industry, which the board believes have made Dr. Vagelos an invaluable resource to both the board and Dr. Schleifer.
The board executes its oversight responsibility for risk management directly and through its Committees,committees, as follows:
•The Audit Committee oversees the Company's risk management program. The Company's Chief Audit Executive,
who reports independently to the Committee, facilitates the risk management program. Audit Committee meetings include discussions of specific risk areas throughout the year, as well as annual reports from the Chief Audit Executive on the Company's enterprise risk profile.
•The Compensation, Corporate Governance and Compliance, and Technology Committees oversee risks associated with their respective areas of responsibility. As part of its overall review of the Company's compensation policies and practices, the Compensation Committee generally considers the risks associated with these policies and practices. The Corporate Governance and Compliance Committee oversees all aspects of the Company's comprehensive compliance program other than financial compliance and considers legal and regulatory compliance risks. The Technology Committee considers risks associated with our research and development programs.•The board is kept abreast of its Committees' risk oversight and other activities via reports of the Committee chairmen to the full board at regular board meetings. The board considers specific risk topics, including risks associated with our strategic plan, our finances, and our development activities. In addition, the board receives detailed regular reports from members of our senior management that include discussions of the risks and exposures involved in their respective areas of responsibility. Further, the board is routinely informed by the appropriate members of senior management of developments internal and external to the Company that could affect our risk profile.
| • | The Audit Committee oversees the Company’s risk management program. The risk management program focuses on the most significant risks the Company faces. The Company’s Chief Audit Executive, who reports independently to the Committee, facilitates the risk management program. Audit Committee meetings include discussions of specific risk areas throughout the year, including, among others, those relating to cybersecurity, and reports from the Chief Audit Executive on the Company’s enterprise risk profile on an annual basis. |
| • | The Compensation, Corporate Governance and Compliance, and Technology Committees oversee risks associated with their respective areas of responsibility. As part of its overall review of the Company’s compensation policies and practices, the Compensation Committee generally considers the risks associated with these policies and practices. The Corporate Governance and Compliance Committee oversees all aspects of the Company’s comprehensive compliance program other than financial compliance and considers legal and regulatory compliance risks. The Technology Committee considers risks associated with our research and development programs. |
| • | The board is kept abreast of its committees’ risk oversight and other activities via reports of the committee chairpersons to the full board at regular board meetings. The board considers specific risk topics, including risks associated with our strategic plan, our finances, and our development activities. In addition, the board receives detailed regular reports from members of our senior management that include discussions of the risks and exposures involved in their respective areas of responsibility. Further, the board is routinely informed by the appropriate members of senior management of developments internal and external to the Company that could affect our risk profile. |
Board Meetings and Attendance of DirectorsEXECUTIVE COMPENSATION PROCESSES AND PROCEDURES; ROLE OF COMPENSATION CONSULTANTS
The board held five regular meetings and two special meetings in 2015. All directors attended more than 75% of the total number of meetings of the board and committees of the board on which they served. According to the Regeneron Board of Directors Corporate Governance Guidelines, board members are expected to attend the Company's Annual Meeting of Shareholders. All of the directors attended our 2015 Annual Meeting of Shareholders, with one director participating through electronic conferencing.
Executive Compensation Processes and Procedures; Role of Compensation Consultants
The Compensation Committee is responsible for overseeing the Company'sCompany’s general compensation objectives and programs. We describe below under “Compensation-Related Matters—Compensation Discussion and Analysis—Compensation Processes” the role of the Compensation Committee, as well as the role of our executive officers, in decisions regarding executive compensation (particularly with respect to our Named Executive Officers referred to(as defined below under "Executive Compensation") below under "Executive Compensation – “Compensation-Related Matters—Compensation Discussion and Analysis – Section 3 – Executive Compensation Process and Considerations – Overview."
19Analysis”)).
Corporate Governance
As discussed in greater detail under "Executive Compensation – Compensation Discussion and Analysis – Section 3 – Executive Compensation Process and Considerations," theThe Compensation Committee has the sole authority to retain its own third-party compensation consultants, and in 20152020 utilized the services of Frederic W. Cook & Co., Inc. ("(“Frederic W. Cook & Co."”), aan independent compensation consultant. Advice and recommendations provided by Frederic W. Cook & Co. may relate to both executive compensation (discussed under "Executive Compensation"in the section “Compensation-Related Matters” below) and director compensation matters (discussed under " – Compensationin the subsection “Compensation of Directors"Directors” below). In addition, managementAs discussed further below, the Corporate Governance and Compliance Committee has adopted a policy requiring the annual review of non-employee director compensation by an independent compensation consultant and separately engaged Frederic W. Cook & Co. for that purpose.
Management also retains another compensation consultant for its own use. In 2015,2020, management used the services of Radford, a compensation consultant focused on the technology and life sciences sectors. Radford provided various consulting services to us, including analyzing the competitiveness of specific compensation programs; preparing surveys of competitive pay practices (including the 2015 Radford Global Life Sciences Survey discussed in this proxy statement);practices; and assisting management in the development and analysis of executive compensation recommendations. Reports prepared by Radford that relate to executive compensation may also be shared with the Compensation Committee.Committee, the full board, or another committee of the board.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 27 / 27 |
OVERVIEW
Overview
The general philosophy we have applied to compensation of our non-employee directors and the Chairman of the Board is similar to the executive compensation philosophy outlined in the Compensation Discussion and Analysis“Compensation-Related Matters” section of this proxy statement. This philosophy, places anincluding its emphasis on equity compensation, inis consistent with the formCompany’s long-term business orientation and has helped ensure alignment of stock options, which reward growth in stock price and align the directors'directors’ interests with those of our shareholders by providing value to the directors only if there is future stock price appreciation and not rendering any value to the directors if the stock price declines below the applicable exercise price. Similar to executive compensation, the emphasis on long-term incentives in the form of stock options has been a consistent part of Regeneron'sRegeneron shareholders.
Non-employee director compensation philosophy and preceded the significant appreciation in Regeneron's stock price that began in early 2011. As discussed in greater detail below, the board of directors voluntarily reduced the number of shares underlying the three most recentmatters are subject to annual stock option awards to the non-employee directors and the Chairman of the Board by 15% each time, consistent with the reductions in annual awards to executive officers implemented in December 2013, 2014, and 2015.
review. The Corporate Governance and Compliance Committee makes recommendations to the board of directors regarding, and the board of directors determines, the compensation of non-employee directors. The Corporate Governance and
Compliance Committee evaluates the appropriate level and form of compensation for non-employee directors at least annually and recommends changes to such compensation to the board of directors when appropriate. Directors who are Company employees receive no additional compensation for serving on our board of directors or its committees. In determining compensation recommendations for the non-employee directors, the Corporate Governance and Compliance Committee considers, among other things, the qualifications, expertise, and demands on our directors, practices of similar companies in the biotechnology industry (including the Peer Group2), and any recommendationscomparative information provided by Frederic W. Cook & Co. In addition, since November 2018, the Corporate Governance and Compliance Committee has required the annual review of non-employee director compensation by an independent compensation consultant, and has engaged Frederic W. Cook & Co. for this purpose in each of the last three years.
The process governing the compensation consultantsarrangements of the Committee or management. Chairman of the Board is described under “Compensation Arrangements of the Chairman of the Board of Directors” below.
The recent changescurrent compensation program for our non-employee directors and the Chairman of the Board (which has been effective for our non-employee directors and the Chairman of the Board since January 2019 and December 2018, respectively, and is further described below) is referred to in this section as the “Current Compensation Program.”3
NON-EMPLOYEE DIRECTOR COMPENSATION PHILOSOPHY
Our philosophy for non-employee director compensation is simple: to attract the most highly qualified directors with a diverse skillset who will serve as stewards of the Company’s long-term prospects and scientific focus. With this in mind, our non-employee director compensation program described below were adopted based on such considerations.
Cash Feesemphasizes equity compensation primarily in the form of stock options, which reward increases in stock price, over cash fees. The board of directors believes that this emphasis is consistent with the Company’s long-term business orientation and Matching Gifthas helped ensure alignment of directors’ interests with those of Regeneron shareholders. Under the Current Compensation Program,
A we have utilized value-denominated equity compensation awards (granted in the form of stock options and a relatively small percentage of RSUs) for our non-employee directors. This feature of the Current Compensation Program is meant to, among other things, ensure greater stability in reported non-employee director receivescompensation on a year-over-year basis. The board of directors believes that the Current Compensation Program is consistent with Regeneron’s philosophy for non-employee director compensation.
| 2 | See “Compensation-Related Matters—Compensation Discussion and Analysis—Compensation Processes—Peer Data” below for a list of the companies included in our Peer Group. |
| 3 | The Current Compensation Program was implemented following the previously disclosed settlement of two shareholder derivative actions (the “Settlement”) and complies with the terms of the Settlement. |
28 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
CASH FEES AND MATCHING GIFT PROGRAM
In 2020, each non-employee director received an annual retainer of $55,000$90,000 and an annual committee retainer of $10,000 for each standing committee of the Company’s board of directors on which the director serves.served. In 2015, the Chairmanaddition, each chairperson of the Audit Committeeeach standing committee received an additional annual retainer of $5,000 (increased$10,000. Compared to $10,000cash compensation of non-employee directors in our Peer Group, the aggregate starting2020 annual retainer for board service and the additional retainers provided to our committee chairpersons were each below the median.4
In addition, as permitted under the Settlement in 2016 as a resultrespect of the change describedannual retainer awarded in the following sentence). Starting in 2016,2021 through 2023, the board of directors approved an(upon the recommendation of the Corporate Governance and Compliance Committee) determined in November 2020 to increase by 5% the limit on the annual retainer. However, the board did not utilize this limit increase for purposes of setting the 2021 annual retainer of $10,000 for each chairperson of the standing committees of the Company's board of directors, which is in addition to the annual retainer payable to all non-employee directors and the annual retainer payable in respect of service as a director on each standing committee (which remained unchanged). kept it unchanged at $90,000.
Non-employee directors are reimbursed for their actual expenses incurred in connection with their activities as directors, which includedinclude travel, hotel, and food and entertainment expenses.expenses (as applicable). In addition, directors are eligible to participate in the Regeneron Matching Gift Program, which was adopted effective January 1, 2013 and is also available to eligible employees. Under this program, the Company matches contributions made by directors and employees to eligible tax-exempt organizations. Effectiveorganizations up to an annual maximum amount of $5,000 per director or employee.
ANNUAL EQUITY AWARDS
2020 and 2021 Equity Awards
The January 1, 2014,2020 and January 2021 annual equity awards to our non-employee directors were made in accordance with the maximum annual match has been increased to $5,000 (from $2,000) per person.
Annual Stock Option Awards
Pursuant toCurrent Compensation Program and complied with the terms ofrespective limits imposed by the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (as well as its predecessor) and resolutionsthe Settlement.
With respect to each of the Compensation Committee adopted on December 16, 2014January 2020 and December 16, 2015, eachJanuary 2021 annual equity awards, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) determined that the targeted aggregate grant date fair value of such equity awards would be set at $600,000 per non-employee director receivesand consist of stock options with a grant date fair value of $480,000 (or 80% thereof) and RSUs with a grant date fair value of $120,000 (or 20% thereof). The January 2020 annual equity awards to our non-employee directors are shown in the table below. On January 4, 2021, each of the then-serving nine non-employee directors received an automaticequity award comprised of stock options representing 3,613 shares of common stock (with a grant date fair value of $480,390) and 248 RSUs (with a grant date fair value of $119,705). The aggregate grant date fair value of the January 2021 equity awards was $600,094 per non-employee director.
Similar to the limit increase described under “Cash Fees and Matching Gift Program” above, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) determined in November 2020 to increase by 5% the annual limit on annual equity compensation per non-employee director set forth in the Settlement as permitted under the Settlement in respect of compensation awarded in 2021 through 2023. However, the board of directors did not utilize this limit increase for purposes of the January 2021 annual equity awards to the non-employee directors discussed above.
Similar to the process undertaken in respect of the January 2020 annual equity awards, the Corporate Governance and Compliance Committee recommended the approval of, and the board of directors approved, the terms of the January 2021 annual equity awards (as well as the limit increase discussed above) after consideration of the review, analysis, and recommendations of Frederic W. Cook & Co. Such analysis focused on, among other matters, the market practices of companies in our Peer Group, other relevant industry and market data points, Regeneron’s non-employee director compensation philosophy (including its emphasis on long-term incentives), and the terms of the Settlement.
| 4 | Based on information reported by our Peer Group companies in 2020. |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 29 / 29 |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
Terms of Equity Awards
The exercise price of a non-employee director stock option to purchase common stock on the first business day of each year, with an exercise priceis equal to the fair market value of a share of common stock on the date of grant (determined for so long as our common stock is listed on the NASDAQ Global Select Market, as the average of the high and low sales price per share of common stock on the NASDAQNasdaq Global Select Market on the date of grant or, if
20
Corporate Governance
such date is not a trading day, on the last preceding date on which there was a sale of the Company'sCompany’s common stock on the NASDAQNasdaq Global Select Market). These
Under the Current Compensation Program, a pro-rata portion of each equity award (i.e., each stock options become exercisable asoption and RSU award) equal to one-thirdthe portion of one year that has passed from its date of grant vests on the date of the sharesCompany’s first annual shareholder meeting following the date of grant, and the remaining portion vests on the first anniversary of the date of grant in eachgrant. The RSU awards contain mandatory deferral provisions, according to which the shares underlying the RSUs will generally not be delivered to the non-employee director until the earliest of (i) the termination of the three subsequent calendar years,non-employee director’s service as a member of the board, (ii) the seventh anniversary of the RSU grant date, and (iii) the date of a change in control (as defined in the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (or its predecessor)). A non-employee director may, subject to compliance with applicable tax rules, elect in writing a maximum deferral period longer than the seventh anniversary of the grant date. Other than as discussed below, the vesting of equity awards is generally subject to continued service on the board, and stock option awards generally expire ten years following the date of grant. In 2015, similar to the reductions in annual awards to executive officers and other employees discussed under "Executive Compensation" below, the Compensation Committee reduced the automatic grant of stock options to our non-employee directors by 15%, from 10,838 shares to 9,212 shares of common stock underlying each such stock option. Similar to the rationale for the reductions in annual awards to executive officers and other employees discussed under "Executive Compensation" below, the impetus for the reductions in the automatic grants to the non-employee directors was to reduce the potential dilutive impact of these grants; the reductions also took into account the increase in the Company stock price since the prior years' awards, with the resulting increases in the grant date fair values of the automatic grants (as determined according to the Black-Scholes model for valuing stock options). The reduced grants to our non-employee directors were made on January 4, 2016. This decrease constituted the third consecutive double-digit percentage decrease in the annual grant of stock options to our non-employee directors, in each case following high stock appreciation, consistent with the reductions in annual awards to executive officers discussed under "Executive Compensation" below.
To the extent they remain unvested and outstanding, stock optionsequity awards granted to a non-employee director continue to vest following the retirement of that director provided applicable conditions relating to the length of the director'sdirector’s service and the director'sdirector’s age have been met. If a non-employee director'sdirector’s service as a member of the board is terminated as a result of his or her death, all of the director's stock optionsdirector’s equity awards will immediately vest in full.
To the extent they remain unvested and outstanding, stock optionsequity awards granted to non-employee directors become fully vested automatically upon a change ofin control of the Company. Each non-employee director has the right to nullify this acceleration of vesting, in whole or in part, if it would cause the director to pay excise taxes under the requirements of the Internal Revenue Code.
Stock Option Awards to New Directors
Starting in 2016, each newEQUITY AWARDS TO NEW DIRECTORS
Under the Current Compensation Program, any newly elected non-employee director will receive an initial stock optionequity award to purchase a number of shareswith an aggregate grant date fair value equal to 5/3rds of the numberaggregate grant date fair value of shares of common stock
underlying the most recent regular annual stock optionequity award to a non-employee director; and, with respect to the annual stock optionequity award to a non-employee director in respect of the first year of his or her service, the numberaggregate grant date fair value of shares of common stock underlying such annual award will be prorated based on the date as of which the non-employee director first becomes a member of the board of directors.
Compensation Arrangements of the Chairman of the Board of Directors
COMPENSATION ARRANGEMENTS OF THE CHAIRMAN OF THE BOARD OF DIRECTORS
On December 31, 1998, we entered into an employment agreement with the Chairman of the board of directors, Dr. Vagelos. Dr. Vagelos did not become an officer of the Company or change his title. Pursuant to the terms of his employment agreement, Dr. Vagelos receives an annual salary of $100,000. In$100,000 as a non-officer employee.
Under the employment agreement, we agreed to recommend to theCurrent Compensation Committee that stock option grants be made toProgram, Dr. Vagelos for calendar years 2000 through 2003 inreceives an annual equity award with an aggregate grant date fair value equal to 10 times the amountaggregate grant date fair value of the greater of (a) 125,000 shares or (b) 125% of the highestcorresponding non-employee director annual option award granted to an officer of the Company.
award. In 2011,December 2020, the Compensation Committee determined that Dr. Vagelos's target grantstock options and RSUs would be equal to ten times the annual grant for a non-employee membercomprise 80% and 20% of the board of directors, setting his target award at 150,000 shares of common stock underlying stock options. In December 2013, 2014, and 2015, the Compensation Committee reduced the2020 annual equity award to Dr. Vagelos, by 15% each time,respectively, and set the targeted aggregate grant date fair value of such award at (but no more than) ten times the aggregate grant date fair value of the corresponding annual equity award for our non-employee directors. As in lineprior years, Dr. Vagelos’s 2020 annual equity award reflects, among other things, the key contributions that Dr. Vagelos makes as the Company continues to gain momentum as a fully integrated biotech company with multiple class-leading products, as well as Dr. Vagelos's crucial role as a trusted advisor to the reductionCEO, other senior managers, and the non-employee directors. It is also designed to incentivize further contributions and ensure Dr. Vagelos's continued service to the Company in the annual stock option awards to our executive officers and the reduction in the annual stock option awards to the non-employee directors made in January 2014, 2015, and 2016. On December 16, 2015, the Compensation Committee granted Dr. Vagelos stock options to purchase 92,123 shares of common stock, at an exercise price of $555.67 per share, the fair market value per share of our common stock on the date of grant (determined as the average of the high and low sales price per share of common stock on the NASDAQ Global Select Market on the date of grant). future.
30 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
The 2020 stock option award granted to Dr. Vagelos vests ratably over four years subject to his continued service and contains change-of-control provisions consistent with those described above for stock optionequity grants to non-employee directors. PursuantThe 2020 RSU award granted to Dr. Vagelos vests 50% on the second anniversary of the date of grant and 50% of the fourth anniversary of the date of grant and contains change-of-control provisions consistent with those described above for equity grants to non-employee directors. In addition, the award agreements relating to Dr. Vagelos’s equity awards granted in 2018-2020 provide that each such award will continue to vest in accordance with the terms of the applicable award agreement following his employment agreement, ifqualified retirement (as defined in the applicable Company policy; Dr. Vagelos dies or is disabled duringcurrently meets the term of his employment, all stock options granted to him by the Company will immediately become vested and exercisable.
21policy requirement).
Corporate Governance
The following table and explanatory footnotes provide information with respect to compensation paid to Dr. Vagelos and each non-employee director for their service in 2015
2020 in accordance with the policies, plans, and employment agreement described above:
Director Compensation
| A | B | C | D | E | F | G | H | |||||||||||||||||||||
| Name | Fees earned or paid in cash ($) | Stock awards1 ($) | Option awards1,2 ($) | Non-equity incentive plan compensation ($) | Change in pension value and non-qualified deferred compensation earnings | All other compensation3 ($) | Total ($) | |||||||||||||||||||||
| Bonnie L. Bassler, Ph.D. | 110,000 | 119,718 | 480,281 | — | — | — | 709,999 | |||||||||||||||||||||
| Michael S. Brown, M.D. | 120,000 | 119,718 | 480,281 | — | — | — | 719,999 | |||||||||||||||||||||
| N. Anthony Coles, M.D. | 100,000 | 119,718 | 480,281 | — | — | 5,0004 | 704,999 | |||||||||||||||||||||
| Joseph L. Goldstein, M.D. | 110,000 | 119,718 | 480,281 | — | — | — | 709,999 | |||||||||||||||||||||
| Christine A. Poon | 120,000 | 119,718 | 480,281 | — | — | — | 719,999 | |||||||||||||||||||||
| Arthur F. Ryan | 120,000 | 119,718 | 480,281 | — | — | 5,0004 | 724,999 | |||||||||||||||||||||
| George L. Sing | 120,000 | 119,718 | 480,281 | — | — | — | 719,999 | |||||||||||||||||||||
| Marc Tessier-Lavigne, Ph.D. | 100,000 | 119,718 | 480,281 | — | — | — | 699,999 | |||||||||||||||||||||
| P. Roy Vagelos, M.D. | — | 1,199,988 | 4,799,997 | — | — | 109,0395 | 6,109,024 | |||||||||||||||||||||
| Huda Y. Zoghbi, M.D. | 110,000 | 119,718 | 480,281 | — | — | — | 709,999 | |||||||||||||||||||||
| 1 | The amounts in columns C and D reflect the respective aggregate grant date fair values of RSUs and options awarded during the year ended December 31, 2020 pursuant to the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan or its predecessor (as applicable). Valuation assumptions and methodologies used in the calculation of these amounts do not take into account expected forfeitures and are otherwise described in Note 12 to the Company’s audited financial statements for the fiscal year ended December 31, 2020 included in the 2020 Annual Report. |
| 2 | At December 31, 2020, the non-employee directors and Dr. Vagelos had the following aggregate number of stock option awards outstanding: Dr. Bassler: 28,257; Dr. Brown: 29,541; Dr. Coles: 28,093; Dr. Goldstein: 6,840; Ms. Poon: 85,744; Mr. Ryan: 6,840; Mr. Sing: 43,464; Dr. Tessier-Lavigne: 56,214; Dr. Vagelos: 973,825; and Dr. Zoghbi: 23,139. |
| 3 | See the subsection "Compensation-Related Matters—Compensation Dashboard—Additional Compensation Information—Perquisites and Personal Benefits" for information regarding director air transportation in accordance with guidelines approved by our board of directors. |
| 4 | Consists of a Company contribution paid or payable on or before April 13, 2021 under the Regeneron Matching Gift Program in respect of charitable gifts made in 2020. |
| 5 | Consists of (i) $103,846 for the salary paid pursuant to the terms of our employment agreement with Dr. Vagelos and (ii) $5,193 for 401(k) Savings Plan matching contributions in respect of 2020. The reported salary gives effect to a 27th pay period in 2020 as a result of the Company’s payroll schedule, which was accelerated to avoid a delayed funds disbursement. This resulted in Dr. Vagelos receiving one additional paycheck in 2020 based on his base salary of $100,000 then in effect. |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 31 / 31 |

Name | | Fees earned or paid in cash ($) (b) | Stock awards ($) (c) | | Option awards ($) 1,2 (d) | Non-equity incentive plan compensation ($) (e) | Change in pension value and non-qualified deferred compensation earnings (f) | | All other compensation ($) (g) | | Total ($) (h) | ||||||||
Charles A. Baker | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Michael S. Brown, M.D. | | 75,000 | – | | 1,986,604 | – | – | | 5,000 | 3 | | 2,066,604 | |||||||
Alfred G. Gilman, M.D., Ph.D.4 | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Joseph L. Goldstein, M.D. | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Robert A. Ingram5 | | 65,000 | | | 1,986,604 | – | – | | – | | 2,051,604 | ||||||||
Christine A. Poon | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Arthur F. Ryan | | 75,000 | – | | 1,986,604 | – | – | | 2,500 | 3 | | 2,064,104 | |||||||
George L. Sing | | 80,000 | – | | 1,986,604 | – | – | | 5,000 | 3 | | 2,071,604 | |||||||
Marc Tessier-Lavigne, Ph.D. | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
P. Roy Vagelos, M.D. | | – | – | | 23,098,558 | – | – | | 109,000 | 6 | | 23,207,558 | |||||||
| | | | | | | | | | | | | | | | | | | | |
1ELECTION OF DIRECTORS
The
amounts in column (d) reflectboard of directors, upon theaggregate grant date fair valuerecommendation ofoptions awarded duringtheyear ended December 31, 2015 pursuant toCorporate Governance and Compliance Committee, has nominated for election at theRegeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Assumptions used in the calculation of this amount do not take into account expected forfeitures2021 Annual Meeting N. Anthony Coles, M.D., Arthur F. Ryan, George L. Sing, andare otherwise described in Note 14 to the Company's audited financial statements for the fiscal year ended December 31, 2015 included in the 2015 Annual Report.
2At December 31, 2015, the non-employee directors and Dr. Vagelos had the following stock option awards outstanding: Mr. Baker: 88,588; Dr. Brown: 35,588; Dr. Gilman: 38,588; Dr. Goldstein: 43,588; Mr. Ingram: 0; Ms. Poon: 93,118; Mr. Ryan: 24,338; Mr. Sing: 105,588; Dr. Tessier-Lavigne: 52,867; and Dr. Vagelos: 2,270,363.
3Consists of a Company contribution paid or payable on or before April 14, 2016 under the Regeneron Matching Gift Program in respect of a charitable gift made in 2015.
4Dr. Gilman servedMarc Tessier-Lavigne, Ph.D. asa director until his death on December 23, 2015.
5Mr. Ingram served as a director until his resignation on November 10, 2015.
6Consists of (i) $100,000 for the salary paid pursuant to the terms of our employment agreement with Dr. Vagelos, (ii) $4,000 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016, and (iii) $5,000Class III Directors for aCompany contribution paid or payable on or before April 14, 2016 underthree-year term expiring at theRegeneron Matching Gift Program in respect of a charitable gift made in 2015.
22
Corporate Governance
![]()
Executive Officers of the Company2024 Annual Meeting.
The board of directors unanimously recommends a vote FOR the election of each of these nominees.
32 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY | |||
EXECUTIVE OFFICERS OF THE COMPANY
All officers of the Company are appointed annually and serve at the pleasure of the board of directors. The names, positions, ages, and background of the Company'sCompany’s executive officers as of April 14, 201613, 2021 are set forth below. Except as identified below, thereThere are no family relationships between any of our directors and executive officers. None of the corporations or other organizations referred to below with which an executive officer has previously been employed or otherwise associated is a parent, subsidiary, or affiliate of the Company.
LEONARD S. SCHLEIFER, M.D., Ph.D., 63, co-founded the Company in 1988, has been a Director
 | Leonard S. Schleifer, M.D., Ph.D.,68, founded the Company in 1988, has been a director and its President and Chief Executive Officer since its inception, and served as Chairman of the Board from 1990 through 1994. Dr. Schleifer, together with Regeneron’s founding scientist, Dr. Yancopoulos, built and has managed the Company over the past 33 years. Dr. Schleifer received his M.D. and Ph.D. in Pharmacology from the University of Virginia. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology. | |
 | George D. Yancopoulos, M.D., Ph.D.,61, joined Dr. Schleifer in 1989 as founding scientist of the Company, and together they built and have managed the Company since then. Dr. Yancopoulos is currently President and Chief Scientific Officer, and has served on the board since 2001. He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and/or developer of the nine FDA-approved drugs the Company has developed, EYLEA, Praluent, Dupixent, Kevzara, Libtayo, Evkeeza, Inmazeb, ZALTRAP, and ARCALYST, as well as of its foundation technologies, including the TRAP technology, VelociGene®, and VelocImmune®. | |
 | Christopher Fenimore,50, has been Senior Vice President, Controller since January 2021. He previously served as Vice President, Controller from March 2017 to December 2020, as Vice President, Deputy Controller from January 2017 to March 2017, and as Vice President, Financial Planning from January 2012 to December 2016. Prior to joining the Company in 2003, he was Vice President, Finance at Mojave Therapeutics, Inc. Mr. Fenimore’s prior experience includes working as a supervising senior accountant at KPMG, as well as healthcare industry-focused venture capital and investment banking roles. Mr. Fenimore holds an M.A. in Biotechnology from Columbia University, an M.B.A. in Professional Accounting from Rutgers Business School, and a B.A. in Economics from Rutgers University. Mr. Fenimore is a Certified Public Accountant in the State of New York. | |
 | Robert E. Landry,57, has been Executive Vice President, Finance since January 2019 and Chief Financial Officer since October 2013. From September 2013 to December 2018, he served as Senior Vice President, Finance. Previously, Mr. Landry served as Senior Vice President, Treasurer, at Pfizer Inc. from October 2012 to August 2013 and Senior Vice President – Finance, Pfizer’s Diversified Business, from October 2009 to October 2012. Prior to those roles, Mr. Landry held a number of positions at Wyeth, which was acquired by Pfizer Inc. in October 2009, including Treasurer and Principal Corporate Officer from 2007 to 2009, Director of Pharmaceutical Marketing and Sales of Wyeth’s Australian affiliate from 2006 to 2007, and Chief Financial Officer of Wyeth’s Australian and New Zealand affiliates from 2004 to 2006. Mr. Landry holds a B.B.A. in Accounting from the University of Notre Dame. | |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 33 / 33 |
| THE COMPANY / EXECUTIVE OFFICERS OF THE COMPANY |
 | Joseph J. LaRosa,62, has been Executive Vice President, General Counsel and Secretary since January 2019. From September 2011 to December 2018, he served as Senior Vice President, General Counsel and Secretary. Before joining Regeneron, Mr. LaRosa was Senior Vice President, General Counsel, and Secretary at Nycomed US Inc. Mr. LaRosa’s prior experience includes working in a number of senior legal positions at Schering-Plough Corporation from 1993 to 2009, where he was a corporate officer and served most recently as Vice President, Legal Affairs, and a member of the Operations Management Team. Mr. LaRosa received his J.D. from New York University School of Law. | |
 | Marion McCourt,61, has been Executive Vice President, Commercial since January 2021. She previously served as Senior Vice President, Commercial from February 2018 to December 2020. From April 2017 until joining the Company, Ms. McCourt served as the Principal Operating Officer and the Chief Operating Officer and President of Axovant Sciences, Inc. Ms. McCourt previously served as chief operating officer of Medivation, Inc. from February 2016 until its acquisition by Pfizer Inc. in September 2016. Previously, Ms. McCourt worked at Amgen Inc., where she most recently served as a Vice President in U.S. Commercial Operations from February 2014 to January 2016. From May 2013 to January 2014, Ms. McCourt served as Vice President and General Manager at Amgen where she was responsible for the bone health and primary care business unit. From 2012 to 2013, she was Chief Operating Officer for AstraZeneca U.S., a division of AstraZeneca plc. Her responsibilities included oversight and leadership of all U.S. commercial functions, including medical affairs, business development, finance, human resources, legal, operations, and corporate affairs. During her 12-year tenure at AstraZeneca, Ms. McCourt was President and Chief Executive Officer of AstraZeneca Canada Inc. from 2011 to 2012 and also held various other roles at AstraZeneca Pharmaceuticals LP, a subsidiary of AstraZeneca plc. Ms. McCourt received her B.S. in Biology from Lafayette College. | |
 | Andrew J. Murphy, Ph.D.,63, has been Executive Vice President, Research since January 2019. He previously served as Senior Vice President, Research, Regeneron Laboratories from January 2013 to December 2018, as Vice President, Target Discovery from May 2005 to December 2012, as Vice President, Gene Discovery and Bioinformatics from January 2001 to May 2005, and Director of Genomics and Bioinformatics from May 1999 to December 2000. Dr. Murphy is a co-inventor of several of the Company’s key technologies, including VelociGene® and VelocImmune®, and continues to lead several technology centers and therapeutic focus areas. He received his B.S. in Molecular Biology at the University of Wisconsin, and his Ph.D. in Human Genetics from Columbia University, College of Physicians and Surgeons. | |
 | Neil Stahl, Ph.D.,64, has been Executive Vice President, Research and Development since January 2015. He previously served as Senior Vice President, Research and Development Sciences from January 2007 to December 2014, as Senior Vice President, Preclinical Development and Biomolecular Sciences from December 2000 to December 2007, and as Vice President, Preclinical Development and Biomolecular Sciences from January 2000 to December 2000. He joined the Company in 1991. Before becoming Vice President, Biomolecular Sciences in 1997, Dr. Stahl was Director, Cytokines and Signal Transduction. Dr. Stahl received his Ph.D. in Biochemistry from Brandeis University. | |
 | Daniel P. Van Plew,48, has been Executive Vice President and General Manager, Industrial Operations and Product Supply since January 2016. From April 2008 to December 2015, Mr. Van Plew served as Senior Vice President and General Manager, Industrial Operations and Product Supply. Prior to that date, he served as Vice President and General Manager, Industrial Operations and Product Supply since joining the Company in 2007. From 2006 until 2007, Mr. Van Plew served as Executive Vice President, R&D and Technical Operations of Crucell Holland B.V., a global biopharmaceutical company. Between 2004 and 2006, Mr. Van Plew held positions of increasing responsibility at Chiron Biopharmaceuticals, part of Chiron Corporation, a biotechnology company, most recently as Senior Director, Vacaville Operations. From 1998 until 2004, Mr. Van Plew held various managerial positions in the health and life sciences practice at Accenture, Ltd., a management consulting business. Mr. Van Plew received his M.S. in Chemistry from The Pennsylvania State University and his M.B.A. from Michigan State University. | |
34 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
Regeneron is committed to good corporate governance, which we believe promotes the long-term interests of shareholders, strengthens the accountability of the Board from 1990 through 1994. Dr. Schleifer received his M.D.board of directors and Ph.D. in Pharmacology from the University of Virginia. Dr. Schleifer is a licensed physicianmanagement, and is certified in Neurology by the American Board of Psychiatry and Neurology.
GEORGE D. YANCOPOULOS, M.D., Ph.D., 56, joined the Company in 1989 as its Founding Scientist and is currently President, Regeneron Laboratories and Chief Scientific Officer. While holding leadership positions, Dr. Yancopoulos headed the Company's laboratories and science organization since joining the Company and, in 1998, was named the Company's first Chief Scientific Officer. Dr. Yancopoulos joined the board in 2001. He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientisthelps build trust in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together withCompany. The following chart summarizes key members of his team, is a principal inventor and developer of the four FDA-approved drugsinformation regarding our corporate governance.
| Board and Other Governance Information | 2021* |
| Size of Board | 12 |
| Number of Independent Directors | 9 |
| Number of Female Directors | 3 |
| Number of Directors Diverse by Race or Ethnicity | 4 |
| Separate Chairman and Chief Executive Officer | ✔ |
| Majority Voting in the Election of Directors | ✔ |
| Director Resignation Policy | ✔ |
| Number of Meetings of the Board of Directors Held in 2020 | 11 |
| Executive Sessions of Independent Directors Presided by Chairman of the Corporate Governance and Compliance Committee without Management Present | ✔ |
| Code of Business Conduct and Ethics Applicable to All Employees, Officers, and Directors | ✔ |
| Annual Board and Committee Self-Evaluations | ✔ |
| Stock Ownership Guidelines for Directors and Senior Executives | ✔ |
| Active Shareholder Engagement | ✔ |
| Shareholder Right to Call Special Shareholder Meeting | ✔ |
| * | As of April 13, 2021. |
While the Company has developed, EYLEA®, Praluent®, ZALTRAP®, and ARCALYST®, as well as of its foundation technologies, including the TRAP technology,VelociGene®, andVelocImmune®.
MICHAEL ABERMAN, M.D., 45, has been Senior Vice President, Strategy and Investor Relations since January 2015. From March 2010 to December 2014, he served as Vice President, Strategy and Investor Relations. Prior to joining the Company, he spent six years as a Wall Street analyst covering the biotechnology industry. From March 2006 until joining the Company, he was Director and Senior Biotechnology Analyst at Credit Suisse. Prior to that, from March 2004 until March 2006, he worked as a Biotechnology Analyst at Morgan Stanley, Inc. From February 2002 through March 2004, Dr. Aberman was Director of Business Development at Antigenics Inc., an oncology-focused biotechnology company. Dr. Aberman received his M.D. with honors from the University of Toronto and his M.B.A. from the Wharton School of the University of Pennsylvania.
ROBERT E. LANDRY, 52, has been Senior Vice President, Finance since September 2013 and Chief Financial Officer since October 2013. Previously, Mr. Landry served as Senior Vice President, Treasurer, at Pfizer Inc. from October 2012 to August 2013 and Senior Vice President – Finance, Pfizer's Diversified Business, from October 2009 to October 2012. Prior to those roles, Mr. Landry held a number of positions at Wyeth, which was acquired by Pfizer Inc. in October 2009, including Treasurer and Principal Corporate Officer from 2007 to 2009, Director of Pharmaceutical Marketing and Sales of Wyeth's Australian affiliate from 2006 to 2007, and Chief Financial Officer of Wyeth's Australian and New Zealand affiliates from 2004 to 2006.
JOSEPH J. LAROSA, 57, has been Senior Vice President, General Counsel, and Secretary since September 2011. Before joining Regeneron, Mr. LaRosa was Senior Vice President, General Counsel, and Secretary at Nycomed US Inc. Mr. LaRosa's prior experience includes working in a number of senior legal positions at Schering-Plough Corporation from 1993 to 2009, where he was a corporate officer and served most recently as Vice President, Legal Affairs, and a member of the Operations Management Team. Mr. LaRosa received his J.D. from New York University School of Law.
DOUGLAS S. McCORKLE, 59, has been Vice President, Controller, and Assistant Treasurer since 2007. Prior to that date, he served as Controller and Assistant Treasurer since 1998. Prior to joining the Company, Mr. McCorkle was Controller of Intergen Company, a manufacturer of biopharmaceutical products, a position he held since 1997. From 1990 to 1996, Mr. McCorkle was employed with Coopers & Lybrand L.L.P., where he specialized in biotechnology clients and served in various positions including Audit Manager from 1995 to 1996. As previously reported, Mr. McCorkle has notified the Company that he plans to retire in early 2017.
PETER POWCHIK, M.D., 59, has been Senior Vice President, Clinical Development since joining the Company in October 2006. Prior to joining the Company, Dr. Powchik was employed at several pharmaceutical companies, serving as Senior Vice President and Chief Medical Officer of Chugai Pharma USA, a position he held from May 2005 until October 2006. From April 2001 until May 2005, he held various senior clinical development positions at Novartis Pharmaceuticals Corporation, most recently as Vice President, US Clinical Development and Medical Affairs. Dr. Powchik held various clinical development positions with Sepracor Inc. and Pfizer Inc. from October 1996 to April 2001.
23
Executive Officers of the Company
Dr. Powchik received his M.D. from New York University School of Medicine.
NEIL STAHL, Ph.D., 59, has been Executive Vice President, Research and Development since January 2015. He previously served as Senior Vice President, Research and Development Sciences from January 2007 to December 2014, as Senior Vice President, Preclinical Development and Biomolecular Sciences from December 2000 to December 2007, and as Vice President, Preclinical Development and Biomolecular Sciences from January 2000 to December 2000. He joined the Company in 1991. Before becoming Vice President, Biomolecular Sciences in July 1997, Dr. Stahl was Director, Cytokines and Signal Transduction. Dr. Stahl received his Ph.D. in Biochemistry from Brandeis University.
ROBERT J. TERIFAY, 56, has been Executive Vice President, Commercial since January 2016. From February 2007 to December 2015, he served as Senior Vice President, Commercial. Prior to joining the Company, Mr. Terifay was employed at several biopharmaceutical companies. From January to October 2006, Mr. Terifay served as President and Chief Operating Officer of Arginox Pharmaceuticals. Prior to his employment at Arginox, Mr. Terifay was Senior Vice President, Business Operations at Synta Pharmaceuticals from March to December 2005. From February 2002 until March 2005, he held various senior commercial and marketing positions at Millennium Pharmaceuticals, Inc., most recently as Senior Vice President, Oncology Commercial. Mr. Terifay was Vice President, Marketing at Cor Therapeutics, Inc. from 1996
until its acquisition by Millennium Pharmaceuticals, Inc. in February 2002. Mr. Terifay was Executive Vice President of Strategic Services at Saatchi & Saatchi, an advertising firm, from 1993 to 1996. From 1985 to 1993, he held various commercial and marketing positions at G.D. Searle & Company. Mr. Terifay received his Master of Management degree in Marketing and Health Service Management from the J.L. Kellogg Graduate School of Management, Northwestern University.
DANIEL P. VAN PLEW, 43, has been Executive Vice President and General Manager, Industrial Operations and Product Supply since January 2016. From April 2008 to December 2015, Mr. Van Plew served as Senior Vice President and General Manager, Industrial Operations and Product Supply. Prior to that date, he served as Vice President and General Manager, Industrial Operations and Product Supply since joining the Company in 2007. From 2006 until 2007, Mr. Van Plew served as Executive Vice President, R&D and Technical Operations of Crucell Holland B.V., a global biopharmaceutical company. Between 2004 and 2006, Mr. Van Plew held positions of increasing responsibility at Chiron Biopharmaceuticals, part of Chiron Corporation, a biotechnology company, most recently as Senior Director, Vacaville Operations. From 1998 until 2004, Mr. Van Plew held various managerial positions in the health and life sciences practice at Accenture, Ltd., a management consulting business. Mr. Van Plew received his M.S. in Chemistry from The Pennsylvania State University and his M.B.A. from Michigan State University.
24
Executive Officers of the Company
![]()
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth, as of April 14, 2016, the number of shares of the Company's Class Adual-class stock, and common stock beneficially owned by each of the Company's directors, each of the Named Officers referred to below under "Executive Compensation," all directors and executive officers as a group, and each other person or group of persons known by the Company to beneficially own more than 5% of the outstanding shares of common stock or Class A stock, based upon (unless indicated otherwise) information obtained from such persons, and the percentage that such shares represent of the number of outstandingno new shares of Class A stock and common stock, respectively.
The Class A stock is convertible on a share-for-share basis into common stock. The Class A stock is entitled to ten votes per share and the common stock is entitled to one vote per share. We have determined beneficial ownershipbeen issued since our initial public offering in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, we believe, based on the information furnished or otherwise available to us, that the persons named in the table below have sole voting1991, and investment power with respect to all shares of Class A stock and common stock shown as beneficially owned by them, subject to applicable community property laws. We have based our calculation of percentage of shares of a class beneficially owned on
1,913,136 shares of Class A stock and 103,165,457 shares of common stock outstanding as of April 14, 2016, except that for each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock beneficially owned by that person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person assume the conversion on April 14, 2016 of all shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group) into common stock and also that no other shares of Class A stock beneficially owned by others are so converted.
In computing the number of shares of common stock beneficially owned by a person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person (and by directors and executive officers as a group), shares of common stock subject to options held by that person (and by directors and executive officers as a group) that are exercisable as of April 14, 2016 or are exercisable within sixty days after April 14, 2016 are deemed to be outstanding. Such shares are not deemed to be outstanding, however, for the purpose of computing the percentage ownership of common stock of any other person.
25
Security Ownership of Certain Beneficial Owners and Management
| | Shares of Class A Stock Beneficially Owned1 | | Shares of Common Stock Beneficially Owned1 | ||||||||
| | | | | | | | | | | | |
Name and Address of Beneficial Owner | | Number | Percent of Class | | Number2 | Percent of Class | |||||
Beneficial Owners of 5% or More of Common Stock or | |||||||||||
| | | | | | | | | | | | |
Sanofi3 | | — | — | | 23,353,665 | 22.6% | |||||
54, rue La Boetie | | | |||||||||
75008 Paris | | | |||||||||
France | | | |||||||||
| | | | | | | | | | | | |
Capital World Investors4 | — | — | 6,804,465 | 6.6% | |||||||
333 South Hope Street | |||||||||||
Los Angeles, California 90071 | |||||||||||
| | | | | | | | | | | | |
FMR LLC5 | | — | — | | 6,223,092 | 6.0% | |||||
245 Summer Street | | | |||||||||
Boston, Massachusetts 02210 | | | |||||||||
| | | | | | | | | | | | |
BlackRock, Inc.6 | — | — | 5,382,473 | 5.2% | |||||||
55 East 52nd Street | |||||||||||
New York, New York 10055 | |||||||||||
| | | | | | | | | | | | |
Directors and Executive Officers:7 | | | |||||||||
| | | | | | | | | | | | |
Leonard S. Schleifer, M.D., Ph.D. | 1,726,565 | 8 | 90.3% | 3,993,844 | 9 | 3.7% | |||||
| | | | | | | | | | | | |
P. Roy Vagelos, M.D. | | — | — | | 2,875,335 | 10 | 2.7% | ||||
| | | | | | | | | | | | |
George D. Yancopoulos, M.D., Ph.D. | 42,750 | 11 | 2.2% | 2,967,079 | 12 | 2.8% | |||||
| | | | | | | | | | | | |
Charles A. Baker | | 62,384 | 13 | 3.3% | | 148,497 | 14 | * | |||
| | | | | | | | | | | | |
Michael S. Brown, M.D. | — | — | 47,462 | 15 | * | ||||||
| | | | | | | | | | | | |
Joseph L. Goldstein, M.D. | | — | — | | 40,113 | 16 | * | ||||
| | | | | | | | | | | | |
Christine A. Poon | — | — | 82,433 | 17 | * | ||||||
| | | | | | | | | | | | |
Arthur F. Ryan | | — | — | | 53,363 | 18 | * | ||||
| | | | | | | | | | | | |
George L. Sing | — | — | 224,385 | 19 | * | ||||||
| | | | | | | | | | | | |
Marc Tessier-Lavigne, Ph.D. | — | — | 42,579 | 20 | * | ||||||
| | | | | | | | | | | | |
Robert E. Landry | | — | — | | 41,656 | 21 | * | ||||
| | | | | | | | | | | | |
Neil Stahl, Ph.D. | — | — | 405,164 | 22 | * | ||||||
| | | | | | | | | | | | |
Robert J. Terifay | | — | — | | 197,466 | 23 | * | ||||
| | | | | | | | | | | | |
All Directors and Executive Officers as a Group (18 persons) | 1,831,699 | 95.7% | 11,720,333 | 24 | 10.4% | ||||||
| | | | | | | | | | | | |
*Represents less than 1%
1The inclusion herein of any Class A stock or common stock, as the case may be, deemed beneficially owned does not constitute an admission of beneficial ownership of those shares.
2For each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock listed includesthe number of shares of Class A stocklistedoutstanding has been steadily decreasing since that time, from 10.9 million to 1.8 million asbeneficially ownedof the record date for the 2021 Annual Meeting.The board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers, and directors. You can find links to this code on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page. We may satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or a waiver from, a provision of our code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or controller, or persons performing similar functions, by posting such
person (or personsinformation on our website where it is accessible through the same link noted above.SUCCESSION PLANNING AND TALENT DEVELOPMENT PROCESS
Under our Corporate Governance Guidelines, the board of directors is required to periodically review with our CEO Regeneron’s plan for succession to the offices of the CEO and other senior executive positions. In 2017, the Corporate Governance and Compliance Committee, at the request of the board of directors, commenced a multi-year formal
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 35 / 35 |
| THE COMPANY / CORPORATE GOVERNANCE |
succession planning and talent review, which includes succession planning for the CEO and other senior management positions and incorporates diversity, equity, and inclusion (“DE&I”) considerations as a strategic priority. From 2018 through 2020, the applicable committees of the board of directors advanced this formal review by focusing on certain assigned functions and roles within the Company. As part of this process, the Audit Committee reviewed functions and roles within the Company’s finance, information technology, and real estate & facilities management organizations, while the Compensation Committee and the Technology Committee reviewed functions and roles within the Company’s commercial and certain other general and administrative organizations and the Company’s research & development and global development organizations, respectively. Implementation of the succession planning and talent review plan has continued in 2021.
In addition to formal succession planning, directors also have exposure to Regeneron leaders through board and committee presentations and discussions and informal events and interactions with key talent throughout the caseyear, both in small-group and one-on-one settings.
Regeneron’s mission is to use the power of science to repeatedly bring new medicines to patients. We are committed to operating responsibly, communicating transparently about our impacts, and engaging all stakeholders in our mission. We strive to “Do Well by Doing Good” and have been publicly disclosing information about significant corporate responsibility matters, including by publishing our annual responsibility reports.
Our board of directors and executive officers asmanagement team focus closely on corporate responsibility matters. The charter of the board’s Corporate Governance and Compliance Committee expressly delegates board oversight of corporate responsibility to the Committee. The Company’s policy is to take into consideration the long-term interests of the Company, its shareholders, and other stakeholders, including patients, employees, the healthcare community, regulators, partners, suppliers, and local communities. Under our Corporate Governance Guidelines, the Corporate Governance and Compliance Committee is responsible for overseeing the Company’s key corporate responsibility initiatives, including those expected to have a group).
3Based solelysignificant impact on the Company’s ability to deliver sustained growth. The Committee also conducts an annual review of environmental, social, and governance (“ESG”) matters. Management, who has the responsibility for formulating and implementing such initiatives and matters, has established for these purposes aForm 4 filed by SanofiResponsibility Committee comprised of cross-functional business leaders.Our responsibility strategy centers on three focus areas:
• Improve the lives of people with serious diseases • Foster a culture of integrity and excellence • Build sustainable communities As shown below, our global 2025 responsibility goals (announced in 2020 in our 2019 Responsibility Report) span across these three focus areas and the environmental and social issues that we believe are most significant to our business and stakeholders.
36 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY / CORPORATE GOVERNANCE |
2025 GLOBAL RESPONSIBILITY GOALS
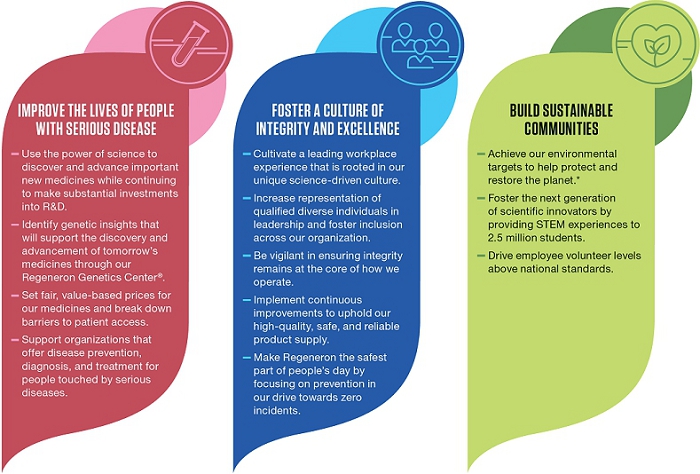
* See our 2020 Responsibility Report for a discussion of our specific environmental targets and highlights of our progress achieved in 2020.
In 2020, we made significant progress toward achieving many of these responsibility goals. Select highlights within each focus area are summarized below, and a detailed discussion of our progress in 2020 is included in our 2020 Responsibility Report.
Our responsibility efforts and results across these focus areas have garnered recognitions. For example, in 2020, we were proud to have been included in the Dow Jones Sustainability World Index for the second year in a row and added to the Dow Jones Sustainability North America Index for the first time. These global and regional indices are comprised of corporate leaders in ESG practices.
We also continued to enhance our reporting of these responsibility efforts and results. In 2021, we published on our website our first report on climate-related risks and opportunities, including how we address management and oversight of these risks and opportunities, aligned with the SEC on February 18, 2016. According to this Form 4, 20,554,113recommendations of the shares are held directly by sanofi-aventis Amerique du NordTask Force on Climate-related Financial Disclosures (“TCFD”). In addition, our 2020 Responsibility Report continues to align with the Sustainability Accounting Standards Board (“SASB”) framework.
Improve the lives of people with serious diseases. As a science-focused company, we operate Regeneron with the long-term outlook required to turn rigorous scientific research into important new medicines. All nine of our approved medicines and 2,799,552almost all of the sharesproduct candidates in our clinical pipeline are held directly by Aventis Pharmaceuticals Inc. sanofi-aventis Amerique du Nord is a direct, wholly-owned subsidiary of Sanofi,homegrown – discovered in Regeneron’s labs using our industry-leading, proprietary technologies. Our support for patients extends beyond the labs to disease education and Aventis Pharmaceuticals Inc. is an indirect, wholly-owned subsidiary of sanofi-aventis Amerique du Nord. Pursuantawareness efforts, product support services, and our commitment to the Amendeddrug access and Restated Investor Agreement, dated as of January 11, 2014, byresponsible pricing. We strive to make thoughtful and among Sanofi, sanofi-aventis US LLC, Aventis Pharmaceuticals Inc.,well-informed pricing decisions with fairness and sanofi-aventis Amerique du Nord (collectively, the "Sanofi Parties"),affordability in mind and the Company, the Sanofi Parties have agreed to vote all shares of our voting securities they are entitled to vote from time to time as recommendedguided in this endeavor by our board of directors, except thatwhich is closely involved in and provides oversight of all key pricing determinations.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 37 / 37 |
| THE COMPANY / CORPORATE GOVERNANCE |
In 2020, our commitment to improving lives was highlighted as more than three decades of Regeneron scientific and technological expertise culminated in two breakthrough achievements: the approval of Inmazeb by the U.S. Food and Drug Administration (“FDA”) for the treatment of infection caused by Zaire ebolavirus and an FDA Emergency Use Authorization for REGEN-COV™ (casirivimab with imdevimab), our investigational dual antibody cocktail to the SARS-CoV-2 virus. We are proud of these scientific accomplishments and the potentially life-saving benefits they may electcan deliver to vote proportionallypatients. We are dedicated to making sure these new medicines are available to everyone who needs them, including those in low- and middle-income countries.
Since 2018, we have worked with the votes cast by allWorld Health Organization, FDA, and other global organizations to offer Inmazeb under a compassionate use protocol in response to Ebola outbreaks in affected African countries. In 2020, we established an internal access working group and proactively engaged public health agencies, non-governmental agencies, and others in our industry to ensure continued access to Inmazeb in low- and middle-income countries. Similarly, we are collaborating with Roche to increase global supply of REGEN-COV and share a commitment to support access in low- and middle-income countries through drug donations made in partnership with public health organizations.
Foster a culture of integrity and excellence. Regeneron’s unique culture makes us who we are. Our culture includes our science-led mindset, our high ethical standards, and our unbridled focus on solving big, complex problems. As we continue to grow, we remain committed to making significant investments to attract and retain top talent and promote DE&I within our workforce and our community.
The well-being of our other shareholders with respect to certain change-of-control transactions and to vote in their sole discretion with respect to liquidation or dissolution of Regeneron, stock issuances equal to or exceeding 20% of the then outstanding shares or voting rights of common stock and Class A stock (taken together), and new equity compensation plans or amendments if not materially consistent with our historical equity compensation practices. See "Certain Relationships and Related Transactions – Transactions with Related Persons – Amended and Restated Investor Agreement with Sanofi" for further information regarding the Amended and Restated Investor Agreement with Sanofi.
26
Security Ownership of Certain Beneficial Owners and Management
4Based solely on an amendment to a Schedule 13G filed by Capital World Investors on February 16, 2016. According to this amendment, Capital World Investors, a division of Capital Research and Management Company, has sole voting and dispositive power as to all of the shares reported as beneficially owned. Capital World Investors is deemed to be the beneficial owner of such shares as a result of Capital Research and Management Company acting as investment adviser to various registered investment companies.
5Based solely on an amendment to a Schedule 13G filed by FMR LLC on February 12, 2016. According to this amendment, FMR LLC has sole voting power as to 639,403 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. Abigail P. Johnsonemployees is aDirector,primary focus. In response to theVice Chairman,COVID-19 pandemic, we implemented changes in our business beginning in March 2020 to protect our employees and support appropriate health and safety protocols, including regular COVID-19 testing for designated employees and contractors who remain onsite in our laboratories and manufacturing facilities and work-from-home policies for a significant portion of our employees.We also continue to strengthen our commitment to DE&I. We appointed an interim DE&I leader in July 2020 and hired our permanent Chief DE&I Officer in January 2021 to advance our strategy. We took important steps to build a more diverse and inclusive workforce, such as launching mandatory inclusion trainings and expanding our diversity-focused recruitment efforts in 2020 and 2021 to date. Our DE&I efforts in 2020 also extended to our communities, including launching a double-matching gift campaign under the
Chief Executive Officer,Regeneron Matching Gift Program to support racial equity and justice efforts and establishing a leadership-led taskforce focused on enhancing patient diversity in clinical trials. We also continued to enhance our DE&I disclosures, including by reporting progress and metrics in our 2020 Responsibility Report and publishing consolidated EEO-1 data (data from annual reports submitted to the U.S. Equal Employment Opportunity Commission) on our website.We are equally committed to conducting our business responsibly and ethically. This is demonstrated through the range of policies, practices, and initiatives we have implemented, encompassing areas such as compliance, responsible sales and marketing, ethical clinical trials, responsible supply chain, and product quality and safety.
Build sustainable communities. We believe that our role in creating a healthier world extends beyond creating life-transforming medicines to building a healthy living environment. In 2019, in connection with the selection of our 2025 responsibility goals, we set ambitious medium- and longer-term environmental sustainability targets. We began reporting on our progress toward these goals and targets in our 2020 Responsibility Report.
We also strengthen our communities through strategic philanthropic investments, product donations, and the
President of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the votingpower ofFMR LLC. The Johnson family groupour employees’ talents andall other Series B shareholders have entered intotime. We are ashareholders' voting agreement under which all Series B voting common shares will be voted in accordance with the majority votelong-standing supporter ofSeries B voting common shares. Accordingly, through their ownership of voting common sharesscience, technology, engineering andthe execution of the shareholders' voting agreement, members of the Johnson family may be deemedmath (“STEM”) education, and make major philanthropic investments toform a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies (the "Fidelity Funds") advised by Fidelity Management & Research Company, a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds' Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by the Fidelity Funds' Boards of Trustees.
6Based solely on an amendment to a Schedule 13G filed by BlackRock, Inc. on February 10, 2016. According to this amendment, BlackRock, Inc. has sole voting power as to 4,830,005, shared voting power as to 5,449, sole dispositive power as to 5,377,024,inspire andshared dispositive power as to 5,449 of the shares reported as beneficially owned.
7The address for each director and executive officer is c/o Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707.
8Includes 15,775 shares of Class A stock held in trust for the benefit of Dr. Schleifer's son, of which Dr. Schleifer is a trustee.
9Includes (i) 2,218,771 shares of common stock purchasable upon the exercise of options granted pursuantcelebrate future scientific innovators, including our ten-year, $100-million commitment to the RegeneronPharmaceuticals, Inc. Second AmendedScience Talent Search, the nation’s most prestigious pre-college science andRestated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016;mathematics competition; and(ii) 5,702 shares of common stock held in an account under the Company's 401(k) Savings Plan.
10Includes (i) 1,995,705 shares of common stock purchasable upon exercise of options granted pursuantour five-year, $24-million commitment to the RegeneronPharmaceuticals, Inc. Second AmendedInternational Science andRestated 2000 Long-Term Incentive Plan orEngineering Fair, the world’s largest pre-college science and engineering competition, which commenced in 2020. Overall, STEM education represented approximately 93% of our corporate philanthropy grants made in 2020, not including medical grants and matched funds.
38 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY / CORPORATE GOVERNANCE |
In 2020, despite the personal challenges of the COVID-19 pandemic, our employees mobilized to give back to our communities. Nearly 3,000 Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Planworldwide employees volunteered more than 7,600 hours to local non-profit organizations through our volunteer programs, including our annual Day for Doing Good. For the first time, this company-wide volunteer event was held virtually over the course of a week to allow greater flexibility. We also engaged employees through our Regeneron Matching Gift Program, offering two double-matching gift campaigns — one to support COVID-19 response efforts and the other to support racial equity and justice efforts.
We are proud to have been named to the Civic 50 for the fourth consecutive year and honored for the first time as the sector leader for healthcare. The Civic 50 recognizes the most community-minded companies in the United States.
For more information about our responsibility efforts and results, please refer to the 2020 Responsibility Report available on our website.
We are committed to adhering to the highest ethical standards when engaging in any political activities. Reflecting this commitment, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) has adopted the Company’s Corporate Political Contributions Policy, a formal written policy that, are exercisable or become so within sixty days after April 14, 2016; (ii) 2,290 sharestogether with our code of common stock held in an accountbusiness conduct and ethics, sets forth our policies and procedures on political contributions and political activity. The policy is available on our website at www.regeneron.com under the Company's 401(k) Savings Plan; (iii) 152,964 shares of common stock held in a charitable lead annuity trust, of which Dr. Vagelos is“Transparency & Policies” heading on the trustee; (iv) 92,947 shares of common stock held in a trust for his grandchildren, of which Dr. Vagelos's wife is the trustee; (v) 1,203 shares of common stock held in trusts for his grandchildren, of which Dr. Vagelos and/or his wife are trustees; and (vi) 56,159 shares of common stock and 241,187 shares of common stock held by the Marianthi Foundation and the Pindaros Foundation, respectively, both of which are charitable foundations of which Dr. Vagelos is a director and an officer. Dr. Vagelos disclaims beneficial ownership of the shares held by these charitable foundations.
11Of these shares, 23,367 shares are held in trust for the benefit of Dr. Yancopoulos's children and certain other family members; Dr. Yancopoulos is a trustee of the trust. The remaining 19,383 shares are held in custody for the benefit of Dr. Yancopoulos's children.
12Includes (i) 1,848,756 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 500,000 shares of restricted stock which vest on December 17, 2017; (iii) 5,676 shares of common stock held in an account under the Company's 401(k) Savings Plan; and (iv) 567,976 shares of common stock held in trust for the benefit of Dr. Yancopoulos's children and certain other family members, of which Dr. Yancopoulos is a trustee.
13All shares of Class A stock are held by a limited partnership, of which Mr. Baker is a general partner.
14Includes 77,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
15Includes (i) 24,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; and (ii) 6,000 shares of common stock held by a family charitable foundation of which Dr. Brown is a director and an officer and his wife is a director. Dr. Brown disclaims beneficial ownership of the shares held by this charitable foundation.
16Includes 27,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
17Includes 81,643 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
18Includes 12,863 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
“Responsibility” page.
27
Security Ownership of Certain Beneficial Owners and Management
19Includes (i) 94,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 3,000 shares of common stock held by Mr. Sing's spouse; (iii) 4,500 shares of common stock held by Mr. Sing's spouse as custodian for the benefit of their son; and (iv) 10,000 shares of common stock held in a trust for benefit of Mr. Sing's son.
20Includes 41,392 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
21Includes (i) 34,500 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 5,000 shares of restricted stock which vest on September 9, 2018; and (iii) 57 shares of common stock held in an account under the Company's 401(k) Savings Plan.
22Includes (i) 352,717 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 20,732 shares of common stock held in separate grantor retained annuity trusts of which Dr. Stahl is the trustee; and (iii) 5,621 shares of common stock held in an account under the Company's 401(k) Savings Plan.
23Includes (i) 172,500 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; and (ii) 1,673 shares of common stock held in an account under the Company's 401(k) Savings Plan.
24Includes (i) 7,483,044 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 515,000 shares of unvested restricted stock; and (iii) 28,853 shares of common stock held in an account under the Company's 401(k) Savings Plan.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 39 / 39 |
28
Security Ownership of Certain Beneficial Owners and Management
![]()
Section 16(a) Beneficial Ownership Reporting Compliance
Based solely upon a review of reports filed pursuant to Section 16(a) of the Exchange Act furnished to the Company during or in respect of the fiscal year ended December 31, 2020 and written representations from reporting persons, the Company is not aware of any director, executive officer, or beneficial owner of more than 10%
of our common stock who has not filed on a timely basis any report required by such Section 16(a) to be filed during or in respect of our fiscal year ended December 31, 2015.
29
Section 16(a) Beneficial Ownership Reporting Compliance
![]()
Certain Relationships and Related Transactions, other than as noted below.
Due to administrative oversight, one stock option exercise and sale transaction for Ms. McCourt was not reported timely, resulting in one Form 4 not having been filed for her on a timely basis; and due to administrative oversight resulting in part from communications with the reporting person’s broker, two gifts for Mr. Ryan exempt from Section 16(b) of the Exchange Act were not reported timely, resulting in one Form 5 not having been filed for him on a timely basis.
40 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
Review, Approval, or Ratification of Transactions with Related PersonsREVIEW, APPROVAL, OR RATIFICATION OF TRANSACTIONS WITH RELATED PERSONS
The board of directors has adopted a written policy for the review, approval, or ratification of related person transactions. The Company considers transactions (or a series of related transactions) in which the Company is a participant, the amount involved exceeds $10,000 in any calendar year, and a director, officer, more than 5% holder of our voting securities, any immediate family member of any of the foregoing, or any related entity of any of the foregoing has a direct or indirect material interest to constitute related person transactions. As amended in January 2015, theThe policy provides for a standing pre-approval of transactions with any passive institutional shareholder who holds more than 5% of our voting securities, transactions where all shareholders receive proportional benefits, and certain transactions with Sanofi.Sanofi to the extent such transactions constitute a related person transaction under the policy. With respect to any new transaction that is deemed pre-approved, the Audit Committee receives a summary of each such transaction and retains the ability to require that one or more of such transactions be subject to the standard approval procedures. The policy also requires that the arrangements relating to a permanent, full-time employment of an immediate family member of a director or executive officer hired by the Company be approved in accordance with the policy. In addition, in the event a person is or becomes a director or executive officer of the Company and an immediate family member of such person is a permanent, full-time employee of the Company, no material, outside-of-the-ordinary-course-of-business change in the terms of employment, including compensation, are permitted to be made without the prior approval of the Audit Committee (except, if the immediate family member is himself or herself an executive officer of the Company, any proposed change in the terms of employment are reviewed and approved in the same manner as compensatory arrangements of other executive officers).
The board of directors has determined that the members of the Audit Committee are best suited to review and approve related person transactions. Accordingly, each related person transaction (other than a transaction that is deemed pre-approved as described above) must be reviewed and approved or ratified by the members of the Audit Committee, other than any member of the Audit Committee that has an interest in the transaction. Under the policy, the Chairman of the Audit Committee is delegated the authority to approve certain related person transactions that require urgent review and approval.
When reviewing, approving, or ratifying a related person transaction, the Audit Committee will consider several factors,
including the benefits to the Company, the impact on a director'sdirector’s independence in the event that a director or his/her immediate family is involved in the transaction, the terms of the transaction, and the terms available to unrelated third parties or to employees in general, if applicable. Related person transactions are approved only if the Audit Committee (or the Chairman of the Audit Committee pursuant to delegated authority in the circumstances noted above) determines that they are in, or are not inconsistent with, the best interests of the Company and our shareholders.
Transactions with Related PersonsTRANSACTIONS WITH RELATED PERSONS
Collaborations with Sanofi
As
Prior to the beneficial owner of 23,353,665 shares of common stockcompletion of the Company, or 22.6% of the common stock outstandingSecondary Offering and Stock Purchase (each as of April 14, 2016,defined below), Sanofi iswas considered a related person of the Company. In 2015,May 2020, a secondary offering of 13,014,646 shares of our Common Stock held by Sanofi was completed (the “Secondary Offering”) and we also purchased 9,806,805 shares directly from Sanofi for an aggregate purchase amount of $5 billion (the “Stock Purchase”). Pursuant to the Secondary Offering and Stock Purchase, Sanofi disposed of all of its shares of common stock in Regeneron, other than 400,000 shares that it retained as of the closing of these transactions, and Sanofi ceased to be a related person of the Company effective as of the closing of these transactions.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 41 / 41 |
| THE COMPANY / CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
In 2020, Sanofi funded $145.0 million of our antibody discovery expenses under the Amended and Restated Discovery and Preclinical Development Agreement, and $747.8$954.1 million of our development and other costs (including $157.4$359.4 million of commercialization-related expenses)expenses and $368.0 million in connection with reimbursements for manufacturing commercial supplies) under the Amended and Restated License and Collaboration Agreement.Agreement, as amended (the “Antibody License and Collaboration Agreement”). In addition, in 2015,2020, we also funded $92.6an aggregate of $99.1 million of Sanofi's Phase 3Sanofi’s development costs for Praluent® and sarilumabother costs under the Amended and RestatedAntibody License and Collaboration Agreement. In 2015,addition, in 2020, we and Sanofi shared lossesprofits in connection with commercialization-related activities, which resulted in us receiving $785.2 million of such profits in the aggregate. We also earned a $50.0 million sales-based milestone payment from Sanofi in 2020. Effective April 1, 2020, we and Sanofi amended the Antibody License and Collaboration Agreement to remove Praluent from the agreement such that, among other things, the agreement no longer governs the development, manufacture, or commercialization of Praluent; and we and Sanofi entered into the Praluent Cross License & Commercialization Agreement whereby we, at our sole cost, are solely responsible for Praluent® (which was launched in 2015 following receiptthe development and commercialization of regulatory approvalPraluent in the United States, and Sanofi, at its sole cost, is solely responsible for the European Union)development and sarilumab, which resulted incommercialization of Praluent outside of the United States. Under the Praluent Cross License & Commercialization Agreement, Sanofi pays us funding $240.0 milliona 5% royalty on Sanofi’s net product sales of such costs. In 2016, Sanofi has continued to fundPraluent outside the agreed-upon worldwide research and development expenses incurred by us and Sanofi, we have continued to fund certain Phase 3 development costs, and we and Sanofi have continued to share certain commercialization-related revenues and expenses under the Agreements.United States until March 31, 2032.
In July 2015, we and2020, Sanofi entered into a collaboration to discover, develop, and commercialize antibody-based cancer treatments in the fieldalso funded an aggregate of immuno-oncology (the "IO Collaboration"). The IO Collaboration is governed by an Immuno-oncology Discovery and Development Agreement (the "IO Discovery Agreement"), and an Immuno-oncology License and Collaboration Agreement (the "IO License and Collaboration Agreement"). In connection with the IO Discovery Agreement, Sanofi made a $265.0 million non-refundable up-front payment to us in 2015. Pursuant to the IO Discovery Agreement, we will spend up to $1,090.0 million (the "IO Discovery Budget") to identify and validate potential immuno-oncology
30
Certain Relationships and Related Transactions
targets and develop therapeutic antibodies against such targets through clinical proof-of-concept. Sanofi will reimburse us for up to $825.0 million of these costs, subject to certain annual limits. In connection with the IO License and Collaboration Agreement, Sanofi made a $375.0 million non-refundable up-front payment to us in 2015. Under the terms of the IO License and Collaboration Agreement, the parties will also co-develop our antibody product candidate targeting the receptor known as Programmed Cell Death protein 1, or PD-1 ("REGN2810"). The parties will share equally, on an ongoing basis, development expenses for REGN2810 up to a total of $650.0 million. In 2015, Sanofi funded $29.2$166.2 million of our research and development expenses under the IOAmended and Restated Immuno-oncology Discovery and Development Agreement and $10.8 million under the IOImmuno-oncology License and Collaboration Agreement.
In 2013, we acquired fromaddition, in 2020, Sanofi exclusive rights to antibodies targeting the platelet derived growth factor (PDGF) family of receptors and ligands. In connection with this arrangement, we made a $10.0 million development milestone payment to Sanofi in 2015.
During 2014, Sanofi agreed to fund up to $17.5funded $64.7 million of agreed-upon costs incurred by uscommercialization-related expenses and $8.9 million in connection with expandingreimbursements for manufacturing capacity at our Rensselaer, New York facility,commercial supplies under the Immuno-oncology License and Collaboration Agreement. During 2020, we also recognized $210.6 million of which $13.2 had been received orrevenue that was receivable as of December 31, 2015.previously deferred from upfront payments received.
A description of our antibody collaboration and our IO Collaborationimmuno-oncology collaboration with Sanofi is set forth in Part II, Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" ofNote 3 to our 2015audited financial statements for the fiscal year ended December 31, 2020 included in the 2020 Annual Report under the heading "Liquidity“a. Sanofi—Antibody” and Capital Resources – Collaborations with Sanofi – Antibodies" and "– “a. Sanofi—Immuno-Oncology,"” respectively.
In February 2015, we and Sanofi entered into an amended and restated collaboration agreement relating to ZALTRAP® (ziv-aflibercept) Injection for Intravenous Infusion (the "Amended ZALTRAP® Collaboration Agreement"). Under the terms of the Amended ZALTRAP® Collaboration Agreement, Sanofi is solely responsible for the development and commercialization of ZALTRAP® for cancer indications worldwide. Sanofi bears the cost of all development and commercialization activities and reimburses Regeneron for its costs for any such activities. Sanofi will pay Regeneron a percentage of aggregate net sales of ZALTRAP® during each calendar year, which percentage shall be from 15% to 30%, depending on the aggregate net sales of ZALTRAP® in such calendar year. Regeneron is also paid for all quantities of ZALTRAP® manufactured by it pursuant to a supply agreement through the earlier of 2021 or the date Sanofi receives regulatory approval to manufacture ZALTRAP® at one of its facilities or a facility of a third party. Regeneron is no longer required to reimburse Sanofi for fifty percent of the development expenses that Sanofi previously funded for the development of ZALTRAP® under the original ZALTRAP® collaboration agreement. As a
result of entering into the Amended ZALTRAP® Collaboration Agreement, in 2015, we recognized $14.9 million of collaboration revenue, which was previously recorded as deferred revenue under the original ZALTRAP® collaboration agreement. In addition, during the year ended December 31, 2015,2020, we recorded $38.8$124.7 million of revenue primarily related to (i) revenues earned from Sanofi based on a percentage of net sales of ZALTRAP®Praluent (commencing effective April 1, 2020, as described above) and (ii) revenues earned from SanofiZALTRAP and manufacturing Praluent and ZALTRAP commercial supplies for manufacturing ZALTRAP® commercial supplies.Sanofi.
A description of the Amended ZALTRAP® Collaboration Agreement is set forth in Part I, Item 1. "Business" of our 2015 Annual Report, under the heading "Collaboration Agreements – Collaborations with Sanofi – ZALTRAP."
Amended and Restated Investor Agreement with SanofiSanofi; 2018 Letter Agreement
In January 2014, we entered into an Amended and Restated Investor Agreement with Sanofi.Sanofi, which was subsequently amended effective as of January 7, 2018 by the letter agreement discussed below and as of May 25, 2020 in connection with the Secondary Offering and the Stock Purchase. Pursuant to the agreement,Amended and Restated Investor Agreement, Sanofi has agreed tomust vote its shares retained following the Secondary Offering and the Stock Purchase as recommended by our board of directors, except that it may elect to vote proportionally with the votes cast by all of our other shareholders with respect to certain change-of-control transactions and to vote in its sole discretion with respect to liquidation or dissolution of our company, stock issuances equal to or exceeding 20% of the then outstanding shares or voting rights of common stock and Class A stock (taken together), and new equity compensation plans or amendments if not materially consistent with our historical equity compensation practices.
In addition, upon Sanofi reaching 20% ownership of our then outstanding shares of Class A stock and common stock (taken together), we were requiredis bound by certain “standstill” provisions under the agreement to appoint an individual agreed upon by us and Sanofi to our board of directors. Subject to certain exceptions, we are required to use our reasonable efforts (including recommending that our shareholders vote in favor) to cause the election of this designee at our annual shareholder meetings for so long as Sanofi maintains an equity interest in us that is the lower of (i) the highest percentage ownership Sanofi attains following its acquisition of 20% of our then outstanding shares of Class A stock and common stock (taken together) and (ii) 25% of our then outstanding shares of Class A stock and common stock (taken together). This designee is required to be "independent" of Regeneron, as determined under NASDAQ rules, and not to be a current or former officer, director, employee, or paid consultant of Sanofi. In April 2014, Sanofi notified us that it had reached the 20% ownership threshold and designated Robert A. Ingram as its designee. On April 4, 2014, following recommendation of the Corporate Governance and Compliance Committee, the board of directors elected Mr. Ingram as a director and a member of the Compensation Committee. Mr. Ingram was subsequently elected as a Class I director at our 2014 annual shareholder meeting for a term expiring at the 2016 annual shareholder meeting and resigned on
31
Certain Relationships and Related Transactions
November 10, 2015. In December 2015, Sanofi disclosed in an amendment to its Schedule 13D filed with the SEC its intention to designate a successor designee.
Under the Amended and Restated Investor Agreement, Sanofi also has three demand rights to require us to use all reasonable efforts to conduct a registered underwritten offering with respect to shares of our common stock held by Sanofi from time to time; however, shares of our common stock held by Sanofi from time to time may not be sold until the later of (i) December 20, 2020 or (ii) the expiration of our discovery and preclinical development agreement with Sanofi relating to our antibody collaboration (as amended) if the agreement is extended beyond December 20, 2020. These restrictions on dispositions are subject to earlier termination upon the occurrence of certain events, such as the consummation of a change-of-control transaction involving us or a dissolution or liquidation of Regeneron.
Pursuant to the Amended and Restated Investor Agreement, Sanofi is bound by certain "standstill" provisions, which contractually prohibit Sanofi from seeking to directly or indirectly
exert control of Regeneron or acquiring more than 30% of our Class A stock and common stock (taken together). This prohibition will remain in place until the earliest of (i) the later of the fifth anniversaries of the expiration or earlier termination of our licenseAntibody License and collaboration agreementCollaboration Agreement with Sanofi relating to our antibody collaboration or our ZALTRAP®ZALTRAP collaboration agreement with Sanofi, each as amended; or (ii) other specified events.
Prior to the May 2020 amendment, under the Amended and Restated Investor Agreement Sanofi had the right to designate an independent board member to our announcement recommending acceptanceboard of directors and Sanofi had certain demand rights to require us to use all reasonable efforts to conduct a registered underwritten offering with respect to shares of our common stock held by Sanofi from time to time. These provisions of the Amended and Restated Investor Agreement, among others, were terminated in connection with the Secondary Offering and Stock Purchase.
Effective January 7, 2018, we and Sanofi and certain of Sanofi’s direct and indirect subsidiaries entered into a letter agreement in connection with (i) the increase of the development budget amount for cemiplimab set forth in
42 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY / CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
the Immuno-oncology License and Collaboration Agreement and (ii) the allocation of additional funds to certain proposed activities relating to the development of dupilumab and REGN3500 and non-approval trials of dupilumab (the “Dupilumab/REGN3500 Eligible Investments”). Pursuant to the letter agreement, we agreed, among other things, to grant Sanofi a limited waiver of the lock-up obligations under the Amended and Restated Investor Agreement (which obligations expired on December 20, 2020) in order to allow Sanofi to satisfy in whole or in part (a) its funding obligations with respect to the cemiplimab development costs under the Immuno-oncology License and Collaboration Agreement for the quarterly periods commencing on October 1, 2017 and ending on September 30, 2020 by selling up to 800,000 shares of our shareholderscommon stock directly or indirectly owned by Sanofi and (b) its funding obligations with respect to the costs incurred by or on behalf of the parties to the Antibody License and Collaboration Agreement with respect to the Dupilumab/REGN3500 Eligible Investments for the quarterly periods commencing on January 1, 2018 and ending on September 30, 2020 by selling up to 600,000 shares of our common stock directly or indirectly owned by Sanofi. Under this arrangement, if Sanofi desired to sell shares of our common stock during the term of the letter agreement to satisfy a tender offerportion or exchange offer that, if consummated, would constitute a changeall of control involving us; (iii)its funding obligations for the public announcement of any definitive agreement providing for a change of control involving us; (iv)cemiplimab development and/or Dupilumab/ REGN3500 Eligible Investments, we had the date of any issuanceright to elect to purchase, in whole or in part, such shares from Sanofi. If we did not elect to purchase such shares, Sanofi could sell the applicable number of shares (subject to certain daily and quarterly limits) in one or more open-market transactions.
In 2020, Sanofi elected to sell, and we elected to purchase (either in cash or by issuing a credit towards the amount owed by Sanofi), an aggregate of 249,148 shares of our common stock by us that would resultpursuant to the letter agreement. This arrangement is no longer in another party's havingeffect.
A more than 10%detailed description of the voting powerletter agreement with Sanofi is set forth in the 2020 Annual Report under Part I. Item 1. “Business — Collaboration, License, and Other Agreements — Sanofi.”
Stanford University
Effective September 1, 2016, Marc Tessier-Lavigne, Ph.D. became the President of Stanford University. In 2020, we made payments to Stanford University of approximately $205,000 in the aggregate relating primarily to services provided in connection with certain clinical trials entered into in the ordinary course of business. In 2021 through the end of the first quarter, we made payments to Stanford of approximately $132,000 relating primarily to a medical education fellowship grant.
Indemnification of Directors and Officers
Our Certificate of Incorporation provides that, to the fullest extent permitted under the New York Business Corporation Law, no director or officer of our then outstanding Class A stock and common stock (taken together) unless such party enters into a standstill agreement containing certain terms substantially similarCompany shall be personally liable to the standstill obligationsCompany or its shareholders for monetary damages for any breach of Sanofi;fiduciary duty in such capacity. In addition, our Amended and Restated By-Laws provide that we shall indemnify our directors and certain of our other personnel against expenses (including attorneys’ fees) and certain other liabilities, including judgments, fines, and amounts paid in settlement, arising out of or (v) other specified events, suchincurred as a liquidationresult of legal actions brought or dissolutionthreatened against them by reason of Regeneron.their position in our Company, subject to certain qualifications and provided that each such person acted in good faith, in a manner that they reasonably believed was in our best interest, and, where applicable, not unlawful. Subject to the provisions of our Certificate of Incorporation, our Amended and Restated By-Laws, and the New York Business Corporation Law, we may also advance expenses of the individuals entitled to indemnification.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 43 / 43 |
32
Certain Relationships and Related Transactions
![]()
Proposal No. 2: Ratification of Appointment of Independent Registered Public Accounting FirmINTRODUCTION
The Audit Committee has appointed PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for the fiscal year ending December 31, 2016.2021. PricewaterhouseCoopers LLP (or its predecessor) has audited the Company'sCompany’s financial statements for the past 2732 years.
The board of directors has directed that the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 20162021 be submitted for ratification by the shareholders at the Annual Meeting. Shareholder ratification of the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 20162021 is not required by the Company'sCompany’s charter documents or otherwise, but is being pursued as a matter of good corporate practice. If shareholders do not ratify the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 2016,2021, the board of directors will consider the matter at its next meeting.
PricewaterhouseCoopers LLP has advised the Company that it will have in attendance at the 20162021 Annual Meeting a representative who will be afforded an opportunity to make a statement, if such representative desires to do so, and will respond to appropriate questions presented at the 20162021 Annual Meeting.
Information about Fees Paid to Independent RegisteredPublic Accounting FirmINFORMATION ABOUT FEES PAID TO INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Aggregate fees incurred related to services provided to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 20152020 and 20142019 were:
| | 2015 | | 2014 | 2020 ($) | 2019 ($) | ||||
Audit Fees | $ | 1,721,000 | $ | 1,567,493 | 2,783,504 | 2,257,951 | |||
Audit-Related Fees | | 2,007 | | – | 254,500 | — | |||
| Tax Fees | 72,000 | 5,000 | |||||||
All Other Fees | | 4,637 | | 4,812 | 6,145 | 6,106 | |||
| | | | | | | | | ||
Total Fees | $ | 1,727,644 | $ | 1,572,305 | 3,116,149 | 2,269,057 | |||
Audit Fees. Audit fees in 20152020 and 20142019 were primarily for professional services rendered for the audit of the Company'sCompany’s financial statements for the fiscal year, including attestation services required under Section 404 of the Sarbanes-Oxley Act of 2002, technical accounting consultations related to the annual audit, and reviews of the Company'sCompany’s quarterly financial statements included in its Form 10-Q filings.
Audit-Related Fees.Fees. Audit-related fees in 20152020 were for professional services rendered forin connection with a secondary offering of our common stock by Sanofi, the reviewissuance and sale of senior notes, and the filing of a benefit plan of a foreign subsidiary of the Company.Registration Statement on Form S-8.
Tax Fees. Tax fees in 2020 and 2019 were for tax planning and advisory services.
All Other Fees. All other fees in 20152020 and 20142019 were for an annual subscriptionsubscriptions to a technical accounting database and for professional services rendered to a foreign subsidiary of the Company.resources.
44 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY / AUDIT MATTERS |
The Audit Committee has adopted a policy regarding the pre-approval of audit and permitted non-audit services to be performed by the Company'sCompany’s independent registered public accounting firm, PricewaterhouseCoopers LLP. The Audit Committee will, on an annual basis, consider and, if appropriate, approve the provision of audit and non-audit services by PricewaterhouseCoopers LLP. The Audit Committee has approved a general provision of $75,000 for accounting advisory and other permissible consulting engagements. Management is responsible for notifying the Audit Committee of the status of accounting advisory and other permissible consulting engagements at regularly scheduled Audit Committee meetings and, if the Audit Committee so determines, the general provision is replenished to $75,000. The Audit Committee did not utilize thede minimisexception to the pre-approval requirements to approve any services provided by PricewaterhouseCoopers LLP during fiscal 2015 and 2014.
The board of directors unanimously recommends a vote FOR ratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016.
33
Proposal No. 2: Ratification of Appointment of Independent Registered Public Accounting Firm
![]()
Audit Committee Report2020 or 2019.
The Audit Committee Report below shall not be deemed to be "soliciting material" or to be filed with the SEC or subject to Regulation 14A or 14C under the Exchange Act, or to the liabilities of Section 18 of the Exchange Act. Notwithstanding anything to the contrary set forth in any of the Company's previous filings under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act that might incorporate future filings, including this proxy statement, in whole or in part, the Audit Committee Report below shall not be incorporated by reference into any such filings.AUDIT COMMITTEE REPORT
We have reviewed the audited financial statements of the Company for the year ended December 31, 2015,2020, which are included in the Company'sCompany’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015,2020, and met with both management and PricewaterhouseCoopers LLP, the Company'sCompany’s independent registered public accounting firm, to discuss those financial statements. The Audit Committee has discussed with the Company'sCompany’s independent registered public accounting firm the matters required to be discussed by Auditing Standard No. 16, as adopted bythe applicable requirements of the Public Company Accounting Oversight Board (the "PCAOB"“PCAOB”), which include, among other items, matters related to and the conduct of the audit of the Company's financial statements.Securities and Exchange Commission. The Audit Committee also discussed with the independent registered public accounting firm their independence relative to the Company and received and reviewed the written disclosures and the letter from the independent registered public accounting firm required by PCAOB Rule 3526 (Communication with Audit Committees Concerning Independence).the PCAOB.
Based on the foregoing discussions and review, the Audit Committee recommended to the board of directors that the audited financial statements of the Company for the year ended December 31, 20152020 be included in the Company'sCompany’s Annual Report on Form 10-K for the fiscal year ended December 31, 20152020 for filing with the Securities and Exchange Commission.
We have appointed PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for the fiscal year ending December 31, 2016.2021. This appointment was based on a variety of factors, including PricewaterhouseCoopers LLP'sLLP’s competence in the fields of accounting and auditing.
The Audit Committee
George L. Sing, Chairman
N. Anthony Coles, M.D.
Arthur F. Ryan
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 45 / 45 |
PROPOSAL 2
RATIFICATION OF APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The board of directors unanimously recommends a vote FOR ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2021.
46 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of April 13, 2021, the number of shares of the Company’s Class A stock and common stock beneficially owned by each of the Company’s directors, each of the NEOs referred to below under “Compensation-Related Matters—Compensation Discussion and Analysis,” all directors and executive officers as a group, and each other person or group of persons known by the Company to beneficially own more than 5% of the outstanding shares of Class A stock or common stock, based upon (unless indicated otherwise) information obtained from such persons, and the percentage that such shares represent of the number of outstanding shares of Class A stock and common stock, respectively.
The Class A stock is convertible on a share-for-share basis into common stock. The Class A stock is entitled to ten votes per share and the common stock is entitled to one vote per share. No new shares of Class A stock have been issued since our initial public offering in 1991. We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, we believe, based on the information furnished or otherwise available to us, that the persons named in the table below have sole voting and investment power with respect to all shares of Class A stock and common stock shown as beneficially owned by them, subject to applicable community property laws. We have based our calculation of percentage of shares of a class beneficially owned on 1,848,970 shares of Class A stock and 104,674,240 shares of common stock outstanding as of April 13, 2021, except that for each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock beneficially owned by that person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person assume the conversion on April 13, 2021 of all shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group) into common stock and also that no other shares of Class A stock beneficially owned by others are so converted.
In computing the number of shares of common stock beneficially owned by a person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person (and by directors and executive officers as a group), shares of common stock subject to options, PSUs, RSUs, or other convertible securities (if any) held by that person (and by directors and executive officers as a group) that are exercisable or releasable as of April 13, 2021 or are exercisable or releasable within sixty days after April 13, 2021 are deemed to be outstanding. Such shares are not deemed to be outstanding, however, for the purpose of computing the percentage ownership of common stock of any other person.
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 47 / 47 |
| SHAREHOLDERS |
| Shares of Class A Stock Beneficially Owned1 | Shares of Common Stock Beneficially Owned1 | |||||||||||||||||
| Name and Address of Beneficial Owner | Number | Percent of Class | Number2 | Percent of Class | ||||||||||||||
| Beneficial Owners of More than 5% of Class A Stock or Common Stock (Other than Directors and Executive Officers): | ||||||||||||||||||
| FMR LLC3 | ||||||||||||||||||
| 245 Summer Street | — | — | 11,481,571 | 11.0 | % | |||||||||||||
| Boston, Massachusetts 02210 | ||||||||||||||||||
| BlackRock, Inc.4 | ||||||||||||||||||
| 55 East 52nd Street | — | — | 9,776,186 | 9.3 | % | |||||||||||||
| New York, New York 10055 | ||||||||||||||||||
| The Vanguard Group, Inc.5 | ||||||||||||||||||
| 100 Vanguard Blvd. | — | — | 7,931,220 | 7.6 | % | |||||||||||||
| Malvern, PA 19355 | ||||||||||||||||||
| Capital World Investors6 | ||||||||||||||||||
| 333 South Hope Street | — | — | 7,017,122 | 6.7 | % | |||||||||||||
| Los Angeles, California 90071 | ||||||||||||||||||
| Directors and Executive Officers:7 | ||||||||||||||||||
| Leonard S. Schleifer, M.D. , Ph.D. | 1,726,5658 | 93.4% | 3,939,400 | 9 | 3.6 | % | ||||||||||||
| P. Roy Vagelos, M.D. | — | — | 1,273,957 | 10 | 1.2 | % | ||||||||||||
| George D. Yancopoulos, M.D., Ph.D. | 42,75011 | 2.3% | 2,427,800 | 12 | 2.3 | % | ||||||||||||
| N. Anthony Coles, M.D. | — | — | 30,418 | 13 | 26 | |||||||||||||
| Bonnie L. Bassler, Ph.D. | — | — | 30,571 | 14 | 26 | |||||||||||||
| Michael S. Brown, M.D. | — | — | 44,724 | 15 | 26 | |||||||||||||
| Joseph L. Goldstein, M.D. | — | — | 14,154 | 16 | 26 | |||||||||||||
| Andrew J. Murphy, Ph.D. | — | — | 339,796 | 17 | 26 | |||||||||||||
| Christine A. Poon | — | — | 88,848 | 18 | 26 | |||||||||||||
| Arthur F. Ryan | — | — | 31,954 | 19 | 26 | |||||||||||||
| George L. Sing | — | — | 104,800 | 20 | 26 | |||||||||||||
| Marc Tessier-Lavigne, Ph.D. | — | — | 59,715 | 21 | 26 | |||||||||||||
| Robert E. Landry | — | — | 114,298 | 22 | 26 | |||||||||||||
| Daniel P. Van Plew | — | — | 258,710 | 23 | 26 | |||||||||||||
| Huda Y. Zoghbi, M.D. | — | — | 25,453 | 24 | 26 | |||||||||||||
| All Directors and Executive Officers as a Group (19 persons) | 1,769,315 | 95.7% | 9,691,887 | 25 | 8.7 | % | ||||||||||||
| 1 | The inclusion in this table of any Class A stock or common stock, as the case may be, deemed beneficially owned does not constitute an admission of beneficial ownership of those shares. | ||
| 2 | For each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock listed includes the number of shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group). | ||
| 3 | Based solely on an amendment to a Schedule 13G jointly filed by FMR LLC and Abigail P. Johnson on February 8, 2021. According to this amendment, FMR LLC has sole voting power as to 1,626,608 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. Abigail P. Johnson is a Director, the Chairman, and the Chief Executive Officer of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, as amended (the “Investment Company Act”), to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act (the “Fidelity Funds”) advised by Fidelity Management & Research Company LLC (“FMR Co. LLC”), a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. FMR Co. LLC carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. |
48 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| SHAREHOLDERS |
| 4 | Based solely on an amendment to a Schedule 13G filed by BlackRock, Inc. on February 1, 2021. According to this amendment, BlackRock, Inc. has sole voting power as to 8,779,238 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. | ||
| 5 | Based solely on an amendment to a Schedule 13G filed by The Vanguard Group, Inc. on February 10, 2021. According to this amendment, The Vanguard Group, Inc. has shared voting power as to 188,647, sole dispositive power as to 7,461,968, and shared dispositive power as to 469,252 of the shares reported as beneficially owned. | ||
| 6 | Based solely on an amendment to a Schedule 13G filed by Capital World Investors on February 16, 2021. According to this amendment, Capital World Investors, a division of Capital Research and Management Company, has sole voting as to 6,999,788 of the shares reported as beneficially owned and dispositive power as to all of the shares reported as beneficially owned. | ||
| 7 | The address for each director and executive officer is c/o Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707. | ||
| 8 | Includes 15,775 shares of Class A stock held in trust for the benefit of Dr. Schleifer’s son, of which Dr. Schleifer is a trustee. | ||
| 9 | Includes (i) 1,632,488 shares of common stock purchasable upon the exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan, the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, or the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (collectively, the “Long-Term Incentive Plans”) that are exercisable or become so within sixty days after April 13, 2021; (ii) 74,691 shares of common stock held in a grantor retained annuity trust of which Dr. Schleifer is the trustee; and (iii) 5,848 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 10 | Includes (i) 637,530 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 2,214 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iii) 142,350 shares of common stock held in a charitable lead annuity trust, of which Dr. Vagelos is the trustee; (iv) 47,786 shares of common stock held in a trust for his grandchildren, of which Dr. Vagelos’s wife is the trustee; (v) 3,609 shares of common stock held in trusts for his grandchildren, of which Dr. Vagelos and/or his wife are trustees; and (vi) 54,146 shares of common stock and 39,671 shares of common stock held by the Marianthi Foundation and the Pindaros Foundation, respectively, both of which are charitable foundations of which Dr. Vagelos is a director and an officer. Dr. Vagelos disclaims beneficial ownership of the shares held by these charitable foundations. | ||
| 11 | Of these shares, 23,367 shares are held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members; Dr. Yancopoulos is a trustee of the trust. The remaining 19,383 shares are held in custody for the benefit of Dr. Yancopoulos’s children. | ||
| 12 | Includes (i) 1,098,053 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 5,821 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iii) 376,861 shares held in a grantor retained annuity trust, of which Dr. Yancopoulos is the trustee; (iv) 822,207 shares of common stock held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members, of which Dr. Yancopoulos is a trustee; and (v) 82,108 shares of common stock held in trusts for the benefit of Dr. Yancopoulos’s children. | ||
| 13 | Consists of (i) 29,657 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 14 | Consists of (i) 29,821 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 15 | Consists of (i) 28,625 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021, which are held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (ii) 9,349 shares of common stock held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (iii) 5,000 shares of common stock held in a trust of which Dr. Brown’s spouse is trustee for the benefit of Dr. Brown’s immediate family members; (iv) 1,000 shares of common stock held by a family charitable foundation of which Dr. Brown is a director and an officer and his wife is a director; and (v) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. Dr. Brown disclaims beneficial ownership of the shares referenced in (iii) and (iv). | ||
| 16 | Includes (i) 8,404 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 17 | Includes (i) 278,204 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 37,839 shares of restricted stock (“RSAs”); and (iii) 4,242 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 18 | Includes (i) 87,308 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 19 | Includes (i) 8,404 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 20 | Includes (i) 45,028 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 1,150 shares of common stock held by Mr. Sing’s spouse; (iii) 400 shares of common stock held by Mr. Sing’s spouse as custodian for the benefit of their son; (iv) 3,700 shares of common stock held in a trust for benefit of Mr. Sing’s son; and (v) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 21 | Includes (i) 57,778 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 49 / 49 |
| SHAREHOLDERS |
| 22 | Includes (i) 88,193 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 22,055 RSAs; and (iii) 202 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 23 | Includes (i) 230,751 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 14,555 RSAs; and (iii) 1,028 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 24 | Consists of (i) 24,703 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; and (ii) 750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021 upon termination of service. | ||
| 25 | Includes (i) 5,092,496 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 13, 2021; (ii) 111,594 RSAs; (iii) 6,750 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 13, 2021; and (iv) 26,930 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 26 | Represents less than 1%. |
The Company has established a process for shareholders to send communications to the members of the board of directors. Shareholders may send such communications by mail addressed to the full board, a specific member or members of the board, or a particular committee of the board, at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, Attention: Corporate Secretary. All such communications will be opened by our Corporate Secretary for the sole purpose of determining whether the contents represent a message to our directors. Any contents that are not in the nature of advertising, promotions of a product or service, or patently offensive material will be forwarded promptly to the addressee. In the case of communications to the board or any individual director or group or committee of directors, the Corporate Secretary will make sufficient copies of the contents to send to such director or each director who is a member of the group or committee to which the envelope is addressed.
50 /  | 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION DISCUSSION AND ANALYSIS | 52 |
| Executive Summary | 53 |
| Compensation Program Overview | 54 |
| Our Corporate Culture and Pay Philosophy | 54 |
| 2020 Business Highlights | 54 |
| 2020 Compensation Highlights | 56 |
| Shareholder Engagement and Feedback | 58 |
| How Our Pay Program Works | 59 |
| Key Governance Features | 60 |
| Compensation Program Objectives | 61 |
| Components of Executive Pay: What We Pay and Why We Pay It | 62 |
| Base Salaries | 63 |
| Annual Cash Incentives | 63 |
| Annual Equity Awards | 69 |
| Perquisites and Personal Benefits | 73 |
| Potential Severance Payments | 73 |
| Compensation Processes | 75 |
| Compensation Committee | 75 |
| Management | 75 |
| Shareholder Input and Outreach | 76 |
| Independent Compensation Consultant | 79 |
| Peer Data | 79 |
| Risk Assessment | 81 |
| Tax Implications | 81 |
| COMPENSATION COMMITTEE REPORT | 83 |
| COMPENSATION COMMITTEE INTERLOCKS ANDINSIDER PARTICIPATION | 83 |
| COMPENSATION DASHBOARD | 84 |
| 2020 Executive Compensation Tables | 84 |
| 2020 Summary Compensation Table | 84 |
| 2020 Grants of Plan-Based Awards | 85 |
| Outstanding Equity Awards at 2020 Fiscal Year-End | 86 |
| 2020 Option Exercises and Stock Vested | 88 |
| Post-Employment Compensation | 88 |
| Additional Compensation Information | 92 |
| Annual Cash Incentive | 92 |
| Perquisites and Personal Benefits | 93 |
| Potential Severance Payments | 95 |
| Pay Ratio | 96 |
| Equity Compensation Information | 97 |
| Corporate Governance Aspects of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | 97 |
| Key Equity Metrics | 97 |
| Equity Compensation Plan Information | 98 |
| 2021 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 51 / 51 |